Abstract
Lentiviruses are quite effective gene delivery systems for stable production of genetically engineered human cells. However, prior to using lentivirus to deliver genetic materials to cells of interest, the normal course of production of these lentiviruses involves a lengthy collection, purification, preservation, and quantification process. In this report, we demonstrate the ability for producer HEK293T cells to simultaneously produce lentiviral particles and transduce (i.e., infect) target cells through a membrane-based coculture system in a continuous, real-time mode which negates the need for a separate viral collection and quantification process. The coculture system was evaluated for major design features such as variations in HEK293T seeding density, target cell type densities, as well as membrane porosities to identify key relationships between lentiviral particle production rate and infection kinetics for adherent and suspension cell types. As a proof-of-concept for the creation of an engineered cell immunotherapy, we describe the ability to engineer human T cells isolated from PBMCs under the control of this coculture system in under 6 days with a GFP construct. These studies suggest the capability to combine and more closely automate the transfection/transduction process in order to facilitate well-timed and cost-effective transduction of target cell types. These experiments provide novel insight into the forthcoming transition into improved manufacturing systems for viral production and subsequent cell engineering.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of genetic engineering of cell therapeutics is showing signs of parabolic growth as a new paradigm of medicine. Cell engineering has been supported by three primary viral vector systems (adeno-associated viruses, γ-retroviruses, and lentiviruses) to deliver transgenes with clinical success in a number of disorders ranging from X-Linked Severe Combined Immunodeficiency (SCID-X1), Hemophilia B, and hematological malignancies using commercialized Chimeric Antigen Receptor (CAR)-T cells (Rogers and Herzog 2015; Hacein-Bey-Abina et al. 2014; Porter et al. 2011). Lentiviral vectors, in particular, have also been used in the treatment of rare diseases, including primary immunodeficiencies and neurodegenerative storage diseases (Mukherjee and Thrasher 2013; Aiuti et al. 2013; Cartier et al. 2009; Biffi et al. 2013). Lentiviruses have been optimized over the past several decades to become the preferred viral vector system, due to their ability to transduce both dividing and non-dividing cells, safer integration profile relative to retroviruses and ability to be produced at high vector titer (Merten et al. 2016). Lentiviral vectors attribute their native infectivity to their HIV-1 backbone derivation, as well as through pseudotyping with a VSV-G envelope protein for improved tropism and transfer of genetic material through direct contact between viral vector and target cell surface for a majority of mammalian cell types (Durand and Cimarelli 2011; Farley et al. 2007). Continued use of lentivirus is foreseeable for the important precedents that they have established in creating commercial-grade cellular medicines, such as CAR-T cells.
A current bottleneck in meeting the growing demands for lentiviral vectors is the manufacture and quality control (QC) (Merten et al. 2010, 2016; Ausubel et al. 2012; Sheu et al. 2015; Gandara et al. 2018) of high titer vector in a closed-system (Nasri et al. 2014; Milone and O’Doherty 2018; Rout-Pitt et al. 2018; McCarron et al. 2016). Conventional methods of lentiviral vector production at a small scale first involves a transfection process that adds three or four lentiviral plasmids to a 2D culture system of HEK293T packaging cells. After transfection, viral particles are secreted by packaging cells and collected in the cell culture supernatant. The packaged vector that is collected is further purified, quantified and stored at − 80 °C while QC tests are performed (Ausubel et al. 2012; Milone and O’Doherty 2018; White et al. 2017). At larger scales, the downstream processing of viral vectors is particularly rife with challenges; with an increase in packaging cell culture scale to increase vector production, there is a concomitant increase in the scaled volume to purify and concentrate the vector which can impact vector functionality, yields, and potency (McCarron et al. 2016; Bandeira et al. 2012; Segura et al. 2006; Nasri et al. 2014). Lentiviruses are also inherently unstable with a half-life of 9 to 12 h at 37 °C (Higashikawa and Chang 2001). During frozen storage, which allows for time for valuable QC tests to be performed, the hold temperature of − 80 °C decrease the lentivirus titer by upwards of 20% (Kumru et al. 2018; Rahman et al. 2013). Such drawbacks are a major challenge for a robust and fast downstream processing system to produce high titer vectors for subsequent transduction of cells ex vivo.
In this study, we explored an integrated system for cell engineering where lentiviral particle production and target cell transduction were combined using a coculture system of packaging cells and target cells. A single, low manipulation system that allowed for production of particles and immediate transduction of a target cell in a potentially closed system would minimize the need for a separate viral collection and processing step. Furthermore, coculture with lentiviral particle-producing cells can provide a continuous source of fresh and highly infective lentivirus in a concentrated system of target cells and lentiviral particles. This approach has been historically evaluated using a coculture system for retroviruses (Germeraad et al. 1994), and this study was focused on lentiviruses, engineering system variables, and the versatility of this method. Herein, we report a transwell based system that investigated HEK293T cells to simultaneously produce lentiviral particles and transduce target adherent cancer cell lines, suspensed Jurkat T cells, or primary T cells. Cell densities and membrane properties were thoroughly assessed to identify prototype system specifications for future scale up efforts. These results provide both a proof-of-concept of single step cell engineering platform and also the framework to evaluate scalable solutions for larger scale human cell therapy applications. We conclude this study with a discussion of safety and engineering control considerations using this system since there is no longer an intermediate vector hold step where QC tests, including a viral titer, are typically performed prior to cell engineering.
Results
Lentiviral-secreting HEK293T cells can infect adherent or suspended target cells in transwell system
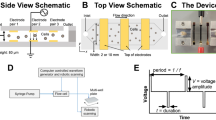
As shown in Fig. 1, the process from the initial seeding of the HEK293T cells until the time at which harvested viral vectors can be used for subsequent transduction of a target cell type, takes on the order of 7–10 days depending on preferred quantification method used (Ausubel et al. 2012; Geraerts et al. 2006) This timeline is greatly extended when considering the full battery of quality testing for lentiviruses before batch release. In order to establish the proof-of-concept that lentiviral particles could be transferred real time within an engineered coculture system containing HEK293T producer cells and a target cell type of interest to engineer, we designed initial experiments to test the feasibility of the system to transduce adherent pancreatic cancer cells (patient 1319) and suspended Jurkat T cells with green fluorescent protein (GFP)-positive lentiviral particles. We additionally wanted to address the possibility of plasmid components permeating through transwell membrane inserts and inadvertently causing target cells to fluoresce. The schematic representation of the engineered coculture system transwell setup is shown in Fig. 2a. We wanted to initially establish a process that could transduce cells in a continuous manner that aligned with the typical timeline of transfection and transduction, shown in Fig. 2b. HEK293T cells, a conventional cell line for production of lentiviral particles, were seeded at 45% confluency in 0.4 μm inserts 24 h prior to addition of lentiviral particle packaging plasmids in order to stabilize the cells and reach an approximate density of 90% at start of transfection. Detailed instructions for transfection of HEK293T cells can be found in materials and methods section. Additional wells were set up containing no HEK293T cells and just media in order to determine if bare plasmids would permeate through the transwell membrane and package within the lower compartment where target cells were located. Target cells were seeded 24 h prior to plasmid addition at 1.8 × 105 cells/mL in a 6-well in order to reach a desired transduction confluency of 25–30% at time when HEK293T cells begin to produce lentiviral particles (between day 24 and 48 as shown in Fig. 2b). After plasmid addition on day 0, plates were incubated for 24 h until media in transwell inserts was changed to collection media (DMEM/F12 only) at which point HEK293T cells began producing progeny lentiviral particles (24–72 h in Fig. 2b). At 48 h, 8 μg/mL polybrene transduction reagent was added to all plates, and Jurkat T cell plates were centrifuged at 2400 rpm for 2 h at 32 °C in a benchtop centrifuge to mimic the standard spinoculation protocol for suspension cells. After centrifugation, cells were replated in fresh complete media, incubated and allowed to grow out for an additional 48 h. Flow cytometry was used to assess transduction efficiency by analyzing GFP expression. GFP positive HEK293T cells and Jurkat T cells are shown in Fig. 2c1, c2, respectively.
Lentivirus secreting HEK293T cells can indirectly infect Jurkat T cells through a membrane system. a Schematic of transduction strategy in a transwell system. HEK293T cells seeded in transwell inserts are constantly producing lentiviral particles over a 48 h period during which target cells in the lower well are being transduced. b Simultaneous timeline of both the transfection and transduction process within the transwell system. Particles are being produced within the 24–72 h time marks. c1 GFP expressing HEK293T cells inside of transwell insert, confirming that transient transfection and therefore lentiviral particle production is occurring. Scale bar: 200 μm. c2 Bottom well containing Jurkat T cells, showing GFP positive cells confirming transduction of target cells from free floating lentiviral particles. Scale bar: 50 μm. See also Figure S1
For all control wells containing HEK293T cells in insert and Jurkat T cells in the well, we saw positive GFP expression from microscopy images. Wells that contained the transwell insert and plasmids, but no HEK293T cells seeded to package those plasmids, showed no GFP expression in bottom cell wells confirming that HEK293T cells were needed in order to successfully package the plasmids and produce progeny lentiviral particles. This also confirmed that target cell viability in the bottom well was not affected by exposure to excessive amounts of free floating DNA. Microscopy images from experiments involving adherent 1319 cells can be found in Supplementary Figure 1. Based off of the qualitative data available for these experiments, there is potential for this system to be used with adherent cell types. Future variation experiments involved Jurkat T cells only to model the more clinically relevant immune cell types which are being evaluated for cell immunotherapy applications.
Cell transduction is dependent on HEK293T and target cell seeding densities
Once confirmation of transduction of target immune cells in a transwell was made, we wanted to assess the different system variables of the engineered coculture that could be varied in order to evaluate their impact on quality and quantity of transduction. The first parameter varied was the density of cells that were seeded in order to determine if the confluency of the HEK293T cells or the Jurkat T cells had an impact on how well the cells could produce lentiviral particles/be infected with particles. HEK293T cells and Jurkat T cells were seeded so that they would be no more than 70–80% confluent at the time of transfection and transduction, respectively (Nasri et al. 2014). Given the doubling time of HEK293T cells, they were seeded a day prior to plasmid addition at lower densities to reach target confluencies of either 30%, 45%, 60%, 75%, or 90% on the day of plasmid addition (transfection) in 0.4 μm transwell inserts. For these groups, Jurkat T cells were seeded at 30% confluency to remain under 70–80% confluency at time of transduction. Flow cytometry was used to determine the effect of varying HEK293T cell density in each insert on the transduction efficiency of target Jurkat T cells, as shown in Fig. 3a, b. In separate plates, Jurkat T cell density in wells was also varied to achieve confluencies of 20%, 30%, 40% and 50% on the day of transduction to assess effect on transduction efficiency, while HEK293T cells in each insert were maintained at 90% confluency, as shown in Fig. 3d, e. Figure 3c represents an image of Jurkat T cells in the well which achieved the highest transduction efficiency (%GFP) which was when HEK293T cells were seeded at 30% confluency to reach a density of 60% at the time of transfection. This level of transduction efficiency is similar to when viral particles are directly used to transduce Jurkat T cells. Supplementary Figure 2 and Fig. 3 represent the images from each well (HEK293T cells) and insert (Jurkat T cells) taken at varying HEK293T and Jurkat cell densities for comparison. HEK293T cells seeded at 60% confluency in the inserts at time of transfection showed a significantly higher level of Jurkat T cell transduction then those seeded at 45% confluency. Seeding HEK293T cells at a confluency of 60% on day 0 proved to be confluent enough at the time of transfection to produce particles, while also not being overconfluent which would deter the transfer of lentiviral particles into the underlying well as seen with the 90% and 75% groups which would presumably come from caking of the transwell pores with a dense cell layer and inhibiting viral particle transport. HEK293T cells seeded at 45% and 30% confluency appeared to not be confluent enough at the time of transfection to produce highly infective particles. Mean fluorescence intensity (MFI) for FITC expression was determined for each HEK293T seeding density, shown in Fig. 3b, and although there was an average increase from control there it was not significant. Briefly, MFI was calculated using the mean of the FITC channel peak for each sample analyzed using flow cytometry. The mean of the FITC channel was used to quantify the shift in GFP expression as determined by the MFI over the MFI from the control value which is at a baseline of 1 for each triplicate (0% GFP expression). Represented as fold increase in each figure as shown. Jurkat density did not seem to have a strong influence on the transduction efficiency when varied as shown in Fig. 3d. Viral particles directly cultured with Jurkat cells showed low GFP expression, while the 20% and 30% groups showed higher efficiency as compared with the 40% and 50% groups, but to no significant degree. Figure 3e shows a slightly significant decrease in MFI from the 10% and 25% seeding density groups as compared to the viral particle control. Figure 3e is an image of the Jurkat cells seeded at 15% confluency to start. Overall the largest influence in transduction efficiency came from the density at which HEK293T cells were seeded in the insert, most likely due to that influencing the ability of viral particles to permeate through the membrane.
Varying cell densities of HEK293T cells in the transwell insert (0.4 μm) and Jurkat T cells in each well to assess impact transduction efficiency. a Transduction efficiency as determined by flow cytometry when the seeding density of HEK293T cells in the 0.4 μm insert was varied. Significant improvements in transduction efficiency in Jurkat T cells was seen when HEK293T cells were seeded to reach 60% confluency at time of transfection over the group where cells were seeded to reach 90% confluency. Results are represented as mean ± SD and one way ANOVA was used to determine significance (p < 0.05). (N = 3) for each data set. b Fold increase in MFI as determined by FITC channel on flow cytometry. No groups were significant. c Fluorescent image of transduced Jurkat T cells when HEK293T cell density in the insert was 60%. Scale bar: 50 μm. See also Figure S2 and Figure S3. d Transduction efficiency as determined by flow cytometry when the seeding density of Jurkat T cells in the well are varied. No groups showed a significantly higher transduction efficiency than others. Results are represented as mean ± SD and one way ANOVA was used to determine significance (p < 0.05). (N = 3). e Fold increase in MFI as determined by FITC channel on flow cytometry. Viral particle (VP) control group was significantly higher than 10% and 25% Jurkat seeding density, and was comparable to the transduction efficiency achieved at 30% confluency. f Fluorescent image of Jurkat cells when seeded at 15% confluency in the wells. Scale bar: 50 μm
Variation in transwell membranes identifies an optimal pore size range
An additional variable affecting engineered coculture system efficiency is the porosity of the transwell insert used to separate the producer HEK293T cells from the target immune cells in the well. Initial experiments were run with 0.4 μm transwell inserts, because that size allowed for passage of ~ 100 nm sized viral particles while limiting the flow through of any cells or other debris. However, we hypothesized that the porosity of the insert could have influence the ability of viral particles to permeate through to lower target cells. To test this, we varied porosity from 0.4, 1, 3 and 8 μm. Flow cytometry analysis was used to determine transduction efficiency for each group as shown in Fig. 4. Microscopy images from porosity groups 3 μm and 8 μm is shown in Fig. 4a, b, and images for the porosity groups 0.4 μm and 1 μm can be found in Supplementary Figure 4. Overall, all membrane pore sizes were similar in achieving transduction of target Jurkat T cells in the well, however there was a significant improvement in the transduction seen in the 3 μm insert compared to the 0.4 μm and 8 μm inserts. Although 8 μm pore size allowed for generation of GFP-positive Jurkat T cells, there were a number of HEK293T cells found in the bottom wells along with Jurkat T cells as shown in Fig. 4b2. The larger 8 μm pore size allowed for some permeation of HEK293T cells through the membrane, which would pose a risk to downstream applications. As shown in Fig. 4c, d, there was a significant increase in MFI in all groups compared to a control, as well as a significant improvement in transduction efficiency in the 3 μm group. Since the 3 μm group was also able to safely limit HEK293T cell migration while also allowing for particle transfer, this size would be chosen for future experiments.
Varying transwell insert porosity in the engineered coculture system. a1, a2 ZEISS flourescent images from 3 μm insert well showing GFP positive HEK293T cells in the insert (a1) and GFP positive Jurkat T cells in the well (a2). Scale bar: 50 μm. b1, b2 ZEISS flourescent images from 8 μm insert. Scale bar: 200 μm. HEK293T cells that permeated through the membrane into the bottom well are shown in b2 which is undesirable for downstream applications with the target immune cell type. Scale bar: 50 μm. c Fold increase in MFI results showing significant increase in all pore size groups over the control (Jurkat cells alone). d Transduction efficiency of Jurkat T cells as determined by flow cytometry results when the porosity of each insert is varied. Results are represented as mean ± SD and one way ANOVA was used to determine significance (p < 0.05). (N = 3 for each data set). See also Figure S4. The 3 μm pore size showed significant increase in transduction efficiency over the 0.4 μm and 8 μm groups
Verification of transduced primary human T cells in a transwell system
Once the major system variables of the engineered coculture system were specified for impact on efficacy of target immune cell transduction, we wanted to validate the system for a widely used target human cell type for engineered cell therapy, T cells, to determine whether this system could successfully transduce these cells with a GFP construct in less than 6 days. In order to assess the effectiveness of the transwell system to transduce a target human cell type, T Cells were isolated from three separate donor PBMCs using preferential magnetic cell separation (detailed procedure can be found in materials and methods section). Isolated T cells were seeded at 1 × 106 cells per mL in a 24 well format, and HEK293T cells were seeded to reach 60% confluency on day of transfection in 3 μm inserts a day prior to plasmid addition. T cell groups were set in triplicate and stimulated with IL-2 1 day prior to transfection on day cells were seeded. Results from flow cytometry are shown in Fig. 5a, b, along with accompanying images of HEK293T cells in inserts in Fig. 5c, d. Lentiviral particles directly cultured with T cells were used as a control group to mimic standard transduction in a 24-well format for these cells as a comparison as detailed in materials and methods and described as viral particles (VP). As shown, transduction efficiency in the T cell group from patient P215 resulted in nearly 40% transduction efficiency comparative to the VP control group. All patient T cell groups showed transduction efficiency > 20% after 5 days in the system. Results from Fig. 5 suggest the potential for the transwell system to transduce key human cell types without compromising cell viability or interfering with downstream processing of the engineered cells. Cell viability was determined using flow cytometry at time transduction efficiency was determined for all experimental groups, and can be found in Supplementary Figure 5.
Transduction of PBMC-isolated T cells in engineered coculture system. a Fold increase in MFI was significantly greater in VP, P215 and PE patient groups compared to the control (Jurkat cells alone). b Transduction efficiency of T cells isolated from 3 separate patients (N = 3) in system after seeding HEK293T cells at 30% confluency in 3 μm inserts. Results are represented as mean ± SD and one way ANOVA was used to determine significance (p < 0.05). Viral particles (VP) to mimic standard transduction in wells with free floating lentiviral particles was used as a control group. Viral particles were combined with isolated T cells at an MOI of 30. There was a significant increase in transduction efficiency between VP control group and patient PE and PV, and patient P215 had a comparable transduction efficiency to VP control. All groups expressed > 20% transduction efficiency is transwell system after 5 days. c, d Fluorescent image of HEK293T cells in transwell insert. GFP positive cells showing successful transfection occurred. Scale bar: 200 μm
Discussion
Lentiviral vectors are a promising reagent for cell engineering because of their potential for a long-lasting treatment of a range of diseases as well as their high levels of gene transfer efficiency and specificity (Milone and O’Doherty 2018) New manufacturing technologies are desperately needed to realize the value of this vector for broad use. This study evaluated a co-culture system of HEK293T cells and human target cells that integrates viral production and target cell engineering in a single platform to streamline the process. A study performed decades ago explored the potential to use transwell inserts to transfer retroviral based particles into murine hematopoietic cells for reinfusion back into mice (Germeraad et al. 1994). These results showed the potential to develop cell products from transduction using an ectotrophic (GP + E86) and amphotropic (GPenvAm12) packaging cell lines to indirectly engineer cell products. The authors found that they could achieve successful transduction over a week to an 18 day span prior to infusion, and importantly, the engineered cells that were transferred into mice caused no adverse effects. Our work focused on a more widely used packaging cell line, HEK293T, and discovered important system properties, such as cell density and membrane porosity, that require specification for effective cell transduction. We also were able to show the versatility of the system to transduce both suspension and adherent human cell types. These proof-of-concept studies of system properties and human use cases can serve as an initial foundation for scale up considerations. The idea of a single stream and scalable process integrating transfection and transduction technologies has not yet been fully implemented in this study, though hollow-fiber bioreactors are considered as a potential technology to scale this process for application to cell engineering of larger cell numbers (Sheu et al. 2015). A closed system hollow-fiber bioreactor has demonstrated the feasibility to immobilize vector-producing HEK293T cells at sufficient viability and achieve titers after vector concentration comparable to 10-layer cell factories (CF10s), while yielding nearly 3 times the volume (Sheu et al. 2015). The continuous circulation of virus could penetrate a semipermeable membrane, similar to the transwell membrane, to transduce inner-membrane circulating target cells in a closed system. Further evaluation of bioreactor technology is warranted to establish feasibility of this technique at larger scales and with downstream cell purification and QC testing in place for this integrated process.
This new system for integrated transfection and transduction can be useful for pre-clinical studies, though there are many adaptations to consider for any clinical use. The current process uses reagents such as polybrene and FBS that should be minimized, if not removed entirely, for a clinical grade cell therapy process. Furthermore, the centrifugation of the coculture is not practical at larger scales so the evaluation of transport in a continuous flow bioreactor will require process development to assure good vector delivery to target cells. Control of viral transport by flow can also negate the need for polybrene (or any cationic carrier) as well. The membrane integrity of any scaled system to assure no migration of viral-producing cells into a final cell therapy product would require rigorous testing and failure mode analysis. Moreover, the improvement in efficiency using this integrated system does come at the sacrifice of loss of control of precise vector doses applied to a cell batch. A well-understood production rate of a given lentiviral vector in this continuous mode as well as engineering runs to specify coculture time to expose a cell therapy to viral particles with assured viability and acceptable transduction efficiency will be required prior to any clinical product manufacturing runs. Further studies at larger scales that also include important downstream steps such as media exchanges, engineered cell expansion, and cell purification that can further reduce impurities would be necessary to build into the process to truly measure a final engineered cell therapy product that could be used for therapeutic studies (Merten and Wright 2016).
Rigorous QC testing is required for any clinical material and this new coculture system poses a frameshift in when, and on what material, QC testing would be performed (Cribbs et al. 2013; White et al. 2017; Gándara et al. 2018). Typically, viral preparations are thoroughly tested for prior to release of viral vectors for cell engineering in compliance with FDA requirements for safety evaluation of lentiviral vectors. Critical QC tests include of a vector stock include measurement of residual protein, plasmid DNA, large T-antigen or replication-competent lentiviruses (RCLs) present. The potency of the vector product is also tested to assess for functionality (Gándara et al. 2018). By using this co-culture system, QC testing of these critical safety attributes would be redirected to the final cell therapy product, rather than an intermediate vector material, prior to final release of an engineered cell therapy for patient use (Quintarelli et al. 2016). This single-step process is, thus, designed for efficiency and consolidates the testing of vector contaminants into an integrated QC evaluation of the final engineered cell product. We explored the issue of residual plasmid DNA contamination that would result in transient cell expression through a transwell membrane, which was not observed in terms of functional expression in target cells. We also evaluated the transfer of HEK293T cells into the target cell compartment which observed at higher membrane pore sizes. Therefore, further studies at larger scales that also include important downstream steps such as media exchanges, engineered cell expansion, and cell purification will be necessary to assess the risk for transfer of DNA, large T antigen, contamination of RCLs and others on a final cell therapy product.
In summary, we developed a novel platform to combine the key transfection and transduction processes for lentivirus production and cell engineering in order to improve the timeline and quality of lentivirus used to engineer key human cell types. We assessed a variety of cell types, both adherent and suspension, to confirm the feasibility of the system and tested initial parameters to prove the potential for system optimization. We were able to achieve transduced target cells with a reporter construct in under 6 days, compared to the > 2 week timeline as reported in literature. Furthermore, combining the two key processes of transfection and transduction can address many of the current issues associated with timelier and cost-effective cell manufacturing methods using lentiviruses. This report can help future development of closed, semi to fully automated systems for manufacturing of cell therapy products with the intent of minimizing cost, reagent use and time.
Materials and methods
Cell culture
Human embryonic kidney cells expressing a mutant version of the SV40 large T antigen (HEK293T) (ATCC: CRL-3216) and pancreatic cancer cells (patient 1319 from Mass General Hospital Bank) were grown in DMEM/F12 medium (Gibco) supplemented with 10% FBS (Gibco) and 1% penicillin/streptomycin (Gibco). Jurkat T cells (Clone E6-1 (ATCC® TIB-152™) and human peripheral blood mononuclear cells (PBMCs) obtained from the New York Blood Center as leukopacks, isolated following Institutional Review Board (IRB) guidelines, were cultured in RPMI-1640 medium (Gibco) with 10% FBS (Gibco) and 1% penicillin/streptomycin (Gibco). Human T cells isolated from PBMCs were cultured in RPMI-1640 media (Gibco) with 10% FBS (Gibco) and 1% Pen/Strep (Gibco) and stimulated using 100 ng/mL Interleukin 2 (IL-2) and 1000 ng/mL Phytohemagglutinin-L (PHA-L), (Sigma-Aldrich).
T cell isolation
T cells were isolated from PBMCs using Miltenyi Biotec Pan T Cell Isolation Kit (MACS Miltenyi Biotec) following the provided protocol. PBMCs were maintained prior to isolation of T cell subsets in complete RPMI-1640 medium (Gibco) with 10% FBS (Gibco) and 1% penicillin/streptomycin (Gibco). For isolation, manual magnetic labeling was carried out by resuspending cell pellet of 80 million PBMCs in 40 μL buffer. 10 μL Pan T Cell Biotin-Antibody Cocktail per 107 cells was added, mixed and incubated at 4 °C. 30 μL buffer was added followed by 20 μL of Pan T Cell Microbead Cocktail per 107 total cells, mixed and incubated for 10 min again at 4 °C. At this point, we proceeded to manual cell separation by placing LS column in magnetic field of MACS separator, rinsed the column with 3 mL of buffer and applied cell suspension to the column. Flow through was collected which contain unlabeled, enriched T cells. T cells were culture overnight prior to use in any experiments.
Production of lentiviral particles
The lentivirus transfer vector pTK113-CMV-GFP, containing reporter GFP gene, packaging plasmid psPAX2 and envelope plasmid pMD2.G (Addgene) were kindly provided by Dr. Rick Cohen for production of lentiviral particles. Plasmids were prepared in two separate batches at ratios of 3:2:1 as follows (10 μg of gene of interest plasmid (pTK113-CMV-GFP), 6.67 μg psPAX2 and 3.33 μg pMD2.G in DMEM/F12 only media solution in the presence of 3.5 × volume (20 μg) polyethylenimine (PEI) transfection reagent (Sigma-Aldrich). Both plasmid mixtures were vortexed vigorously and let sit for 15 min at room temperature prior to addition to HEK293T cells in transwell experiments or in a 10 cm dish containing 8 × 106 HEK293T cells in 10 mL transfection media [DMEM/F12 + 10% FBS − 1% penicillin/streptomycin (Gibco)], for particle production used as positive control. 24 h later media was changed to DMEM/F12 only collection media (− FBS − Pen/Strep), and 24 and 48 h after the media change, lentiviral particles were harvested from the HEK293T cell culture supernatant, filtered using a 0.45 PES filter, and stored at − 80 °C until needed in transwell experiments.
Transfection of HEK293T cells and lentiviral particle production for cell density studies
Generation of lentiviral particles was accomplished by transfection of HEK293T cells seeded at various densities within the cell inserts depending on each experiment. Cell density studies were done in a 24-well format, meaning 1.71 × 104, 1.43 × 104, 1.14 × 104, 8.55 × 103, and 5.7 × 103 HEK293T cells were seeded in triplicate in 0.4 μm transwell inserts in 300 μL complete media [DMEM/F12 + 10% FBS + 1% penicillin/streptomycin (Gibco)], to reach a confluency of 90%, 75%, 60%, 45% and 30%, respectively, 24 h later on day of plasmid addition. Cell seeding density calculations for experiments were determined based on 0.4 μm transwell insert surface area (0.3 cm2) which is ~ 15.8% the surface area of the 24-well (1.9 cm2) therefore cells were seeded at ~ 15.8% the confluency of a 24-well. 7.2 × 104 Jurkat T cells were seeded in each well in triplicate on day 0 at the same time as the HEK293T cells in 1 mL complete media [RPMI-1640 media (Gibco) with 10% FBS (Gibco) and 1% Pen/Strep (Gibco)] so that transwell inserts would be partially submerged in media. 24 h later, media in each HEK293T cell insert was changed to transfection media [DMEM/F12 + 10% FBS only (Gibco)], − penicillin/streptomycin, and plasmid mixture was added to each insert dropwise. For transfection in 10 cm dishes, ~ 10% total volume is plasmid mix, therefore 33 μL total plasmid mix was added to HEK293T cells in each transwell insert to allow the total volume to be 333 μL. 24 h after plasmid addition, HEK293T cell media in inserts was changed to collection media [DMEM/F12 only (− FBS and − penicillin/streptomycin)] (Gibco) and 8 μg/mL polybrene was added to target cell media in each well. After media was changed, plates were centrifuged in a clinical benchtop centrifuge at 1000×g for 90 min at 32 °C. Plates were then moved to incubator (37 °C and 5% CO2) overnight to allow for transfer of lentiviral particles through the inserts into wells containing target cells. The next day inserts containing HEK293T cells were removed from wells and target cells were resuspended in culture media, spun down at 500×g for 5 min at 4 °C, and replated in 3 mL fresh culture media depending on target cell type in 6-well plates. Cells were allowed to grow out for an additional 48 h before analyzing %GFP expression using flow cytometry.
Transfection of HEK293T cells and lentiviral particle production for porosity studies
Porosity studies were done in triplicate in 6-well format. 1.8 × 105 Jurkat T cells were seeded on day 0 in each well in 2 mL complete RPMI media, and 2.7 × 105, 2.25 × 105, 1.8 × 105, 1.35 × 105, and 9 × 104 HEK293T cells were seeded in 900 μL in 6-well transwell inserts at varying pore sizes from 0.4, 1, 3 and 8 μm (Grenier Bio-One). Similarly to cell density experiments, seeding density was determined based on 6-well transwell insert surface area (4.524 cm2) which is ~ 47% the size of the well in a 6-well plate (9.6 cm2), meaning cells were seeded at ~ 47% the confluency of a 6-well. Membranes are made from polyethylene terephthalate (PET) and were either translucent or transparent depending on size. HEK293T cells were seeded in triplicate to reach confluencies of 90%, 75%, 60%, 45% and 30% on day of plasmid addition 24 h later. 24 h later, media in each HEK293T cell insert was changed to transfection media [DMEM/F12 + 10% FBS only (Gibco)], − penicillin/streptomycin, and 100 μL of total plasmid mixture was added to each HEK293T cell insert dropwise to make the total volume in each insert 1 mL. 24 h after plasmid addition, HEK293T cell media in inserts was changed to collection media [DMEM/F12 only (− FBS and − penicillin/streptomycin)] (Gibco). The day after media was changed, 8 μg/mL polybrene was added to T cells in each well and plates were centrifuged in a clinical benchtop centrifuge at 1000×g for 90 min at 32 °C. Plates were then moved to incubator (37 °C and 5% CO2) overnight to allow for transfer of lentiviral particles through the inserts into wells containing target cells. The next day inserts containing HEK293T cells were removed from wells and target cells were resuspended in culture media, spun down at 500×g for 5 min at 4 °C, and replated in 4 mL fresh culture media depending on target cell type in 6-well plates. Cells were allowed to grow out for an additional 48 h before analyzing %GFP expression using flow cytometry.
Transduction of primary human T cells
Primary human T cell studies were carried out using PBMC isolated T cells from three separate donors (P215, PE and PV). Isolation occurred following protocol above. Cells were seeded at 0.5 × 106 cells per mL in 3 mL complete RPMI-1640 medium (Gibco) and stimulated with 200 IU/mL IL-2. HEK293T cells were seeded at 30% confluency in 3 μm inserts at the same time as the T cells 24 h prior to plasmid addition. 24 h later, media in each HEK293T cell insert was changed to collection media (DMEM/F12 only) and 100 μL of total plasmid mixture was added to each HEK293T cell insert dropwise to make the total volume in each insert 1 mL. 48 h later, 8 μg/mL polybrene was added to T cells in each well and plates were centrifuged in a clinical benchtop centrifuge at 1000×g for 90 min at 32 °C. Plates were then moved to incubator (37 °C and 5% CO2) overnight, and the next day inserts containing HEK293T cells were removed from wells and T cells were resuspended in culture media, spun down at 500×g for 5 min at 4 °C, and replated in 5 mL fresh culture media in 6-well plates. Cells were analyzed for %GFP expression 24 h later.
Fluorescent microscopy
An Axio Observer Light Microscope (ZEISS) microscope was used to qualitatively analyze samples for expression of the green fluorescent protein (GFP).
Flow cytometry
Lentiviral transduction of target cells was analyzed by GFP expression using flow cytometry analysis performed using a BD FACS Canto II and FACS Diva software (BD Biosciences). Data was analyzed using FlowJo software (Tree Star). 50,000 and 100,000 events per sample were collected for each experiment depending on cell type. Positive staining was gated based on negative control cells that did not express GFP.
Statistics
All data were represented as mean ± SD. All experiments were run in triplicate and comparisons between means of each group were made with the use of ordinary one-way ANOVA followed by Tukey’s post hoc test. P < 0.05 was considered significant.
References
Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C et al (2013) Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science 341:1233151
Ausubel L, Hall C, Sharma A et al (2012) Production of CGMP-grade lentiviral vectors. Bioprocess Int 10:32–43
Bandeira V, Peixoto C, Rodrigues AF, Cruz PE, Alves PM, Coroadinha AS, Carrondo MJ (2012) Downstream processing of lentiviral vectors: releasing bottlenecks. Hum Gene Ther Methods 23:255–263
Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T et al (2013) Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 341:1233158
Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I et al (2009) Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 326:818–823
Cribbs A, Kennedy A, Gregory B, Brennan F (2013) Simplified production and concentration of lentiviral vectors to achieve high transduction in primary human T cells. BMC Biotechnol 13:98
Durand S, Cimarelli A (2011) The inside out of lentiviral vectors. Viruses 3:132–159
Farley DC, Iqball S, Smith JC, Miskin JE, Kingsman SM, Mitrophanous KA (2007) Factors that influence VSV-G pseudotyping and transduction efficiency of lentiviral vectors-in vitro and in vivo implications. J Gene Med 9:345–356
Gandara C, Affleck V, Stoll EA (2018) Manufacture of third-generation lentivirus for preclinical use, with process development considerations for translation to good manufacturing practice. Hum Gene Ther Methods 29:1–15
Geraerts M, Willems S, Baekelandt V, Debyser Z, Gijsbers R (2006) Comparision of lentiviral vector titration methods. BMC Biotechnol 6:34
Germeraad WT, Asami N, Fujimoto S, Mazda O, Katsura Y (1994) Efficient retrovirus-mediated gene transduction into murine hematopoietic stem cells and long-lasting expression using a transwell coculture system. Blood 84:780–788
Hacein-Bey-Abina S, Pai SY, Gaspar HB, Armant M, Berry CC, Blanche S, Bleesing J, Blondeau J, de Boer H, Buckland KF et al (2014) A modified gamma-retrovirus vector for X-linked severe combined immunodeficiency. N Engl J Med 371:1407–1417
Higashikawa F, Chang L (2001) Kinetic analyses of stability of simple and complex retroviral vectors. Virology 280:124–131
Kumru O, Wang Y, Gombotz C, Wayne R, Kelley-Clarke B et al (2018) Physical characterization and stabilization of a lentiviral vector against adsorption and freeze–thaw. Pharm Biotechnol 107:2764–2774
McCarron A, Donnelley M, McIntyre C, Parsons D (2016) Challenges of up-scaling lentivirus production and processing. J Biotechnol 240:23–30
Merten O, Wright JF (2016) Towards routine manufacturing of gene therapy drugs. Mol Ther Methods Clin Dev 3:16021
Merten O, Charrier S, Laroudie N et al (2010) Large-scale manufacture and characterization of a lentiviral vector produced for clinical ex vivo gene therapy application. Hum Gene Ther 22:343–356
Merten O, Hebben M, Bovolenta C (2016) Production of lentiviral vectors. Mol Ther Methods Clin Dev 3:16017
Milone M, O’Doherty U (2018) Clincal use of lentiviral vectors. Leukemia 32:1529–1541
Mukherjee S, Thrasher AJ (2013) Gene therapy for PIDs: progress, pitfalls and prospects. Gene 525:174–181
Nasri M, Karimi A, Farsani MA (2014) Production, purification and titration of a lentivirus-based vector for gene delivery purposes. Cytotechnology 66:1031–1038
Porter DL, Levine BL, Kalos M, Bagg A, June CH (2011) Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365:725–733
Quintarelli C, Locatelli F, Caruana I, Angelis BD (2016) Overcoming challenges in CAR T-cell product CGMP release. Mol Ther 24:845–846
Rahman H, Taylor J, Clack B, Stewart RS, Canterberry SC (2013) Effects of storage conditions on the morphology and titer of lentiviral vectors. Faculty Publications. 94
Rogers GL, Herzog RW (2015) Gene therapy for hemophilia. Front Biosci 20:556–603
Rout-Pitt N, McCarron A, McIntyre C, Parsons D, Donnelley M (2018) Large-scale production of lentiviral vectors using multilayer cell factories. J Biol Methods 5:90
Segura MM, Kamen A, Garnier A (2006) Downstream processing of oncoretroviral and lentiviral gene therapy vectors. Biotechnol Adv 24:321–337
Sheu J, Beltzer J, Fury B, Wilczek K, Tobin S, Falconer D, Nolta J, Bauer G (2015) Large-scale production of lentiviral vector in a closed system hollow fiber bioreactor. Mol Ther Methods Clin Dev 2:15020
White M, Whittaker R, Gándara C, Stoll EA (2017) A guide to approaching regulatory considerations for lentiviral-mediated gene therapies. Hum Gene Ther Methods 28:163–176
Acknowledgements
This work was supported in part by the National Institutes of Health Grants (R01GM127353 and R01EB012521).
Author information
Authors and Affiliations
Contributions
LMT and BP formulated the research study design, LMT conducted experiments, acquired data, analyzed data, wrote manuscript. RP assisted in conducting experiments and acquiring data. MT assisted in conducting experiments.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Timmins, L.M., Patel, R.S., Teryek, M.S. et al. Real-time transfer of lentiviral particles by producer cells using an engineered coculture system. Cytotechnology 71, 1019–1031 (2019). https://doi.org/10.1007/s10616-019-00343-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-019-00343-0