Abstract
Endometrial epithelial cells (EECs) cultured in vitro are valuable tools for investigating embryo implantation and trophoblast differentiation. In this study, we have established the bovine EECs and trophoblast stem-like (TS) coculture system, and used it to investigate the binucleate cell formation of ungulates. The EECs was derived from the uterine horn ipsilateral to the corpus luteum by using collagenase I and deoxyribonuclease I, which exhibited typical epithelial morphology and were expressing bovine uterine epithelial marker such as IFNAR1, IFNAR2, Erα, PGR, ESR1 and KRT18. The cells immunostained positively by epithelial and trophectoderm marker cytokeratin 18 (KRT18) and stromal marker vimentin antibodies, and the KRT18 positive cells reached 99 %. The EECs can be cultured for up to 20 passages in vitro with no significant morphology changes and uterine epithelial marker gene expression alteration. The bTS cells were established in a dual inhibitor system and exhibited typical trophoblast stem cell characteristics. When bTS cells were cultured with EECs, the bTS cells adhered to the EECs as adhering to feeder cells. Binucleate cells began appearing on day 4 of coculture and reached approximately 18.47 % of the differentiated cells. Quantitative real-time PCR or immunofluorescence analyses were performed on bTS cells cocultured at day 6 and day 12. The results showed that the expression level of KRT18 was down-regulated while the expression level of trophoblast differentiation marker MASH2, HAND1, GCM1 and CDX2 was up-regulated in bTS cells. In conclusion, bovine EECs can be obtained from the uterine horn ipsilateral to the corpus luteum via treatment with collagenase I and deoxyribonuclease I, and the EECs-bTS cells coculture system presents an ideal tool for studying the differentiation of bTS cells to trophoblast binucleate cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Initiation of embryo implantation involves trophoblast cell adhesion to the epithelial lining of the endometrium. However, the mechanisms regulating the uterine epithelium adhesive properties during the initiation of bovine embryo implantation are still incompletely understood (Thie and Denker 2002). In bovine species, it is difficult to achieve continuous sampling of uterine and conceptus tissues and compounded due to the large cost (Sakurai et al. 2012). Therefore, the development of in vitro models is required to study implantation processes. Bovine endometrial epithelial cells (EECs) are often used as a bovine in vitro model for studying the biological characteristics, cellular and molecular mechanisms, and responses to infection or damage of the bovine uterine epithelium, as well as for investigating the impacts of bovine embryo implantation and development of interactions between the embryo and mother (Bai et al. 2014; Fortier et al. 1988; Hou et al. 2015; Skarzynski et al. 2000; Zhang et al. 2013). Coculture of bovine EECs and bovine trophoblast cells can be used to investigate the maturation of trophoblasts and the formation of binucleate cells. The binucleate cells (BNC) that account for approximately 20 % (15–20 %) of the total trophoblast cells are a distinct population of trophoblastic cells in ruminants (Wooding et al. 1996). The BNCs plays a central role in forming the structures and secretions at the fetomaternal interface that are crucial in establishing and maintaining pregnancy in ruminant. A well-orchestrated transcriptional command from binucleate cells is pivotal to the establishment and progression of pregnancy in cattle (Hashizume et al. 2007).
The isolation and culture of primary bovine EECs have been previously described in several reports (Horn et al. 1998; Skarzynski et al. 2000; Sakurai et al. 2012). But the purity of endometrial cells has not been analyzed. Additionally, immortalized bovine EECs have been established through the transfection of EECs with retroviral vectors encoding human papillomavirus E6 and E7 and human telomerase reverse transcriptase genes (Bai et al. 2014). These established bovine immortalized EECs (imEECs) show the same morphology as primary EECs and retain EEC features, such as exhibiting oxytocin and interferon tau (IFNτ) responsiveness. Therefore, imEECs, even after a number of passages, can be used as an in vitro model to investigate the cellular and molecular mechanisms of the bovine uterine epithelium. However, the side effects of viral transfection require full investigation. Bovine EECs have been used to develop conceptus implantation models over the past several decades (Munson et al. 1991; Sakurai et al. 2012). An in vitro coculture system composed of bovine trophoblast CT-1 cells and primary uterine EECs, which mimics the in vivo attachment process, was established in 2012 (Sakurai et al. 2012), and the resulting findings suggest that this in vitro coculture system can be used to isolate processes that are essential for conceptus attachment to uterine EECs.
In this study, bovine EECs were obtained from the bovine uterine horn ipsilateral to the corpus luteum via treatment with collagenase I and deoxyribonuclease I, the KRT18 positive cells reached 99 %, and used to be cocultured with bTS cells in 2i culture medium. Binucleate cells began to appear on day 4 of coculture and reached approximately 18.47 % of the differentiated bTS cells. The expression level of trophoblast differentiation marker MASH2, HAND1, GCM1 and CDX2 was up-regulated in the differentiated cells. This coculture system is not only an ideal tool for isolation processes that are essential for conceptus attachment but also for studying bTS cell differentiation into binucleated trophoblast cells.
Materials and methods
Chemicals and cell culture conditions
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise indicated. All cells were cultured at 38 °C in a humidified atmosphere of air with 5 % CO2, unless otherwise indicated.
Isolation of endometrial cells
Primary bovine EECs were isolated and cultured as previously described (Horn et al. 1998; Skarzynski et al. 2000) with some modifications. Briefly, the healthy normal whole uteruses were removed from cows at a local abattoir within 30 min of death. The uteruses were quickly transported to the laboratory on ice where the uterine horn ipsilateral to the corpus luteum was dissected from the uterus and uteri of the early estrous cycle (Days 2–5) were used for EECs isolation and culture. A polyvinyl catheter was inserted through the oviduct into the lumen, and the end of the horn near the corpus uteri was sewn up to allow it to retain an enzyme solution (described below) for dissociating the epithelial cells. This part of the uterus was washed five times inside and outside with 30–50 ml of sterile Dulbecco’s phosphate-buffered saline (DPBS) supplemented with 0.1 % penicillin–streptomycin (P.S., Gibco, Grand Island, NY, USA, 15140, Liquid, 100×). A 20-ml sterile injector was used to fill the lumen through the catheter with 50 ml of an enzyme solution composed of sterile DPBS containing 0.5 mg/ml collagenase I (Sigma; C-0130) and 20 KU/ml deoxyribonuclease I (Sigma; D-4263). Epithelial cells were obtained through incubation at 38 °C for 5 h with gentle shaking. The cell suspensions obtained from these digestions were filtered by a metal mesh (80 μm) to remove any tissue fragments that had not been dissociated. The filtrates were then washed three times by centrifugation (5 min at 400×g) with culture medium [DMEM/Ham’s F-12; 1:1 (v:v) (Gibco, 11330-032) containing 15 % FBS (Hyclone, Logan, UT, USA, NWG0445) and 0.1 % P.S.]. Lastly, a hemocytometer was used to count the cells. The resulting cell viability was higher than 81 % as assessed by trypan blue dye exclusion (Trypan Blue: dilute at 0.5 % in DPBS. Procedure: Trypsinize cells and mix cells 1:1 with trypan blue solution then count cells).
Endometrial cell culture and passaging
The freshly isolated cells were centrifuged, and the resulting pellet was resuspended in culture medium. The cells were plated in 60-mm plates (Costar, Cambridge, MA, USA) that had been treated with 0.2 % gelatin (balanced at 38 °C in a humidified atmosphere of air with 5 % CO2 for 1 h; all plates used for cell culture were treated like this, unless otherwise indicated) to allow the cells to attach and grow. To purify the EECs, 1 ml of 0.05 % trypsin was added to the whole plate for 3 min at 38 °C. The plate was subsequently washed three times with DPBS to remove the stromal cells. The culture medium was changed when the cells were attached, and from that point onward, the culture medium was changed every 48 h. The epithelial cells became confluent in 6–8 days and were passaged when they became confluent.
For the passaging of EECs, 1 ml of 0.25 % trypsin was used to treat the cells for 5 min, and then 3 ml of culture medium was added to neutralize the trypsin and the plate was scraped with a cell scraper to obtain the cells that were not removed by the trypsin treatment. The cells were centrifuged for 5 min at 400×g, resuspended in culture medium, and plated in 60-mm plates at a density of 5 × 105 viable cells/ml.
Endometrial cell purity analyses
The purity analyses of EECs consisted of two methods, flow cytometry analysis and counting of KRT18 immunofluorescence positive cells under a microscope.
Flow cytometry analysis: EECs and bovine fetal fibroblast cells (as negative control) were dissociated with trypsin to give a single-cell suspension. Then the cells were incubated with Flow Cytometry Perm Buffer (Tonbo Biosciences, San Diego, CA, USA, RNB-1213-L150, 10×) for 15 min, washed by 10 % goat serum in DPBS for 1 time, and then incubated with Perm Concentrate (Tonbo Biosciences, TNB-1020-L050, 4×) for 15 min and washed by 10 % goat serum in DPBS for 3 times. Then, the cells were incubated with anti-KRT18 antibody for 10 min, and were washed 3 times in DPBS with 10 % goat serum. Finally, the cells were resuspended in DPBS and analyzed by flow cytometer (BD FACSArin™ Special Order Research Project, San Diego, CA, USA) and FACS plots were processed using FlowJo 7.6.1 software.
KRT18 positive cells were calculated as follows: Eight randomly chosen microscopy fields from the KRT18 immunofluorescence stained slides and analyzed and the positive cells were counted. Then the percentage of the KRT18 positive cells was calculated.
Immunofluorescence analyses
We used a polyclonal anti-KRT18 antibody (FITC-conjugated, Sigma, Green) to identify epithelial cells, as well as DAPI (4′,6-diamidino-2-phenylindole; Sigma, Blue) to identify the cell nuclei. To test for the presence of stromal cell contamination, a mouse monoclonal antibody against vimentin (Abcam, Cambridge, U.K.) was used as the primary antibody and it was visualized with a DTAF-conjugated anti-mouse secondary antibody (Invitrogen, Carlsbad, CA, USA; Green).
The immunofluorescence assays were operated as follows: The cells were cultured in 4-well plates (Nunc, Thermo, Roskilde, Denmark) for 6 days, then gently washed once with DPBS for 5 min and then fixed in 300 µl/well 4 % paraformaldehyde for 10 min at room temperature. After three washes with DPBS, they were treated with 1 % Triton X-100 for 10 min to permeate the cell membranes. After washing once with DPBS, the cells were then blocked with 10 % goat serum in DPBS for 30 min at 38 °C. The primary antibodies were added at a 1:100 dilution in 10 % goat serum, and the cells were incubated overnight at 4 °C. Afterward, the cells were washed with DPBS for 3 times. For vimentin staining, the cells were incubated with a goat anti-mouse IgG (Invitrogen) secondary antibody at a 1:300 dilution, in the dark for 1 h at room temperature and washed with DPBS for 3 times. All the cells were treated with 10 ng/ml of DAPI to stain the cell nuclei. Finally, the cells were washed with DPBS, and fluorescent images were acquired with a confocal microscope, either an Olympus (Germany) or Nikon C1 (Japan).
Coculture of bTS and bEECs cells
The bTS cells that had been established using the dual inhibitor system, which consisted of DMEM/Ham’s F-12 and Neurobasal™ Medium (Gibco, 21103-049), with 0.5 μM PD0325901 (STEMCELL, 72182), 3 μM CHIR99021 (STEMCELL, 72052), B-27 Supplement (Gibco, 17504-044, 50×), N-2 Supplement (Gibco, 17502-048, 100×) and Glutamax (Gibco, 35050-061, 100×), with mixed feeders L Wnt-3A/MEF cells (L Wnt-3A cells were purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China, and MEF cells were isolated and stored by our lab) were mixed at a ratio of 1:1 and were treated with mitomycin C (17 mg/mL; Haizheng, Tai Zhou, Zhe Jiang, China) for 2.5–3 h at 37 °C (Huang et al. 2014; Li et al. 2008) were used in coculture with bovine EECs. The culture medium of this system was named 2i culture medium.
EECs were cultured in 4-well plates for 36 h, and then the culture medium (DMEM/Ham’s F-12; 15 % FBS; 0.1 % P.S.) was changed to 2i culture medium. Then, bTS cells (bTS cells need mechanical passaging, the cell colonies were cut into small pieces by the edge of injector needle per well, 0.5 × 0.5 mm2) were transferred into the wells containing the EECs. The 2i cell culture medium was changed 48 h later, after the cells were initially seeded into the plates, and then medium was changed every 24 h. To investigate the effect of EECs on bTS cell differentiation, the cells were analyzed at day 6 and day 12 of coculture. At day 6 of coculture, the cells were fixed in paraformaldehyde and subjected to fluorescence immunohistochemistry to determine the expression levels of cytokeratin and vimentin. At day 12 of coculture, the cells were used for three separate experiments: (1) fluorescence immunohistochemistry to determine the cytokeratin expression levels; (2) microscopy to identify binucleated cells, and the number of binucleate cells was counted in eight randomly chosen microscopy fields; or (3) total RNA extraction from bTS cells followed by real-time quantitative PCR experiments to compare the expression levels of MASH2, HAND1, GCM1, CDX2 and KRT18 between the cocultured cells and separately cultured cells.
Gene expression analysis
To find out the difference between P2 and P20 bEECs and the differentiation marker expression of cocultured bTS cells, real-time PCR was performed.
Total RNA from P2, P20 bEECs, cultured separately and cocultured bTS cells was extracted using the mRNA Trizol (Thermo Fisher Scientific, Waltham, MA, USA) method according to the manufacturer’s instructions. RNA quality and quantity were analyzed using a NanoDrop 2000c Spectrophotometer (Thermo Scientific). The cDNA was obtained by using PrimeScript™ RT reagent kits (Takara, Kusatsu, Shiga, Japan) according to the manufacturer’s instructions. The reactions proceeded at 37 °C for 15 min followed by 85 °C for 5 s, and the resultant products were stored at 4 °C until they were analyzed.
Quantitative real-time PCR: The primers sets used are listed in Table 1. Real-time PCR amplification was conducted using a Jena qTOWER 2.2 real-time PCR System (Analytik Jena AG, Jena, Germany). A KAPA SYBR FAST Universal qPCR kit (KAPA Biosystems, Wilmington, MA, USA) was used according to the manufacturer’s instructions to provide real-time quantification of the desired PCR products. Each real-time PCR reaction mixture contained 1.5 μl cDNA and 0.5 µl of each primer in a total volume of 20 μl. PCR reactions were initiated at 95 °C for 3 min, followed by 40 cycles of 94 °C for 15 s, 58.5 °C for 30 s, and 72 °C for 30 s. Reactions were terminated after a final 10 min at 72 °C. All tests were conducted in triplicate, and the product identity was confirmed by a melting curve analysis. The mRNA levels of genes were normalized to that of mRNA encoding GAPDH.
Results
The derivation of bovine EECs
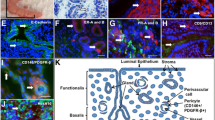
To obtain the bovine EECs, the uterine horn ipsilateral to the corpus luteum was used. The primary EECs were obtained (Fig. 1a) by dissociating the cells with an enzyme solution (0.5 mg/ml collagenase I and 20 KU/ml deoxyribonuclease I) and purified by treating the cells with 0.05 % trypsin and washing with DPBS to remove stromal cells and other impurities during the first passaging. The epithelial cells derived by this method exhibited typical epithelial cell traits, such as cuboidal or columnar morphologies and contact inhibition at confluence (Fig. 1a, b). Totally, 3 cell lines were established. The cultured EECs were able to be passaged at least 20 times without any detectable changes in the cell morphology (Fig. 1a, b). The results of fluorescence immunohistochemistry with the anti-KRT18 antibody showed that 99.3 % of the cultured cells expressed KRT18, which is only expressed by epithelial cells and usually used to identify the EECs. Vimentin, a stromal cell marker, was also detected in all EEC cells by fluorescence immunohistochemistry (Fig. 1g, h) and the EECs stained only with secondary antibody and DAPI were used as control (Fig. 1i, j). The results of flow cytometry analysis showed 99.1 % cells were KRT18 positive. In 88.6 % of EECs (Fig. 2a), 99.1 % cells were stained by FITC-conjugated anti-KRT18 antibody (Fig. 2b), and for bovine fetal fibroblasts cells (Fig. 2c) as control, in 92.5 % cells, only 0.227 % cells were stained (Fig. 2d). The results of the relative quantification of EECs gene expression at different passages showed there was no significant difference of expression level of IFNAR1, IFNAR2, Erα, PGR, ESR1 and KRT18 between P2 and P20 bEECs (Fig. 2e).
Morphology and characteristics of bovine EECs cells. Morphology of bovine primary EECs (a) and bEECs at passage 20 (b). c The KRT18 immunofluorescence staining of bEECs at passage 3. d The merged KRT18 immunofluorescence staining and DAPI of bEECs at passage 3. e KRT18 immunofluorescence staining of bovine fetal fibroblast cells at passage 6 as control. f The merged KRT18 immunofluorescence staining and DAPI of bovine fetal fibroblast cells at passage 6 as control. g The vimentin immunofluorescence staining of bEECs at passage 3. h The merged vimentin immunofluorescence staining and DAPI of bEECs at passage 3. i The immunofluorescence staining of bEECs only with secondary antibody at passage 3 as control. j The merged immunofluorescence staining of EECs only with secondary antibody and DAPI at passage 3 as control. All scale bars represent 100 μm
Flow cytometry analysis of KRT18 staining of bEECs at P2, and the relative quantification of gene expression of bEECs at P2 and P20. The KRT18 positive cell analysis of bEECs at passage 2 is shown in a and b and bovine fetal fibroblast cells at passage 6 are shown in c and d. 1 × 104 cells were analyzed. In 88.6 % gated bEECs (a), 99.1 % cells were stained by FITC-conjugated anti-KRT18 antibody (b). In 92.5 % gated bovine fetal fibroblast cells (c), 0.227 % cells were stained by FITC-conjugated anti-KRT18 antibody (d). e The relative quantification of gene expression of bEECs at different passages (this graph was done by software GraphPad Prism 6), and the results showed that there is no significant difference of expression level of IFNAR1, IFNAR2, Erα, PGR, ESR1 and KRT18 between P2 and P20 bEECs
Coculture of bTS with bEECs cells promote the binucleate cell formation
The bTS cells (Fig. 3a, c, d) were collected and mechanically cut under a microscope. After being plated onto EECs, the bTS cells attached on day 2 of coculture and then expanded and formed distinct colonies by day 6 of coculture. After the bTS cells were plated and cultured with EECs, they attached quickly to the EECs, but the morphology difference of both cell population was still obvious (Fig. 3b). The binucleate cells appeared at day 4 of differentiation. The degree of bTS cell differentiation increased progressingly from the inside to the outside of the colonies (Fig. 3b). Specifically, the morphology of the inner cells resembled that of the original bTS cells: dome-shaped, small, compact, and irregular; at the edge, the border and nuclei of the cells became clearer and the cells became bigger and flatter, which resembled the morphology of binucleated cells. Additionally, the ratio of binucleated cells to cells with a single nucleus was higher near or at the border of the cell colonies.
Coculture of bEECs with bTS cells. a Morphology of bTS cells cultured in dual inhibitor system with mixed feeders. b Morphology of bTS cells cocultured with bEECs. c KRT18 immunofluorescence staining of bTS cells cultured in dual inhibitor system for 6 days. d The 6 days cultured bTS cells merged staining of KRT18 and DAPI. e Immunofluorescence staining of KRT18 of bTS cells after cocultured for 6 days. f The merged staining of KRT18 and DAPI after cocultured for 6 days. g Immunofluorescence staining of KRT18 after cocultured for 12 days. h The merged staining of KRT18 and DAPI after cocultured for 12 days. All scale bars represent 100 μm
We examined the cells at two different stages: cocultured for 6 days and for 12 days. The KRT18 immunofluorescence staining of cells cocultured for 6 days (Fig. 3e, f) and 12 days (Fig. 3g, h) indicated that the cocultured EECs was expressing KRT18, but the number of positive stained cells decreased with the differentiation process. The percentage of binucleate cells to the total number of cells in eight randomly chosen microscopy fields, an example of which is shown in Fig. 4a and b, was 18.47 %, which is similar to the binucleate cell percentage in vivo. The results from the quantitative real-time PCR assay of bTS cells cocultured for 12 days showed that the relative expression level of the epithelial cell marker KRT18 was decreased while the trophoblast differentiation markers MASH2, HAND1, GCM1 and CDX2 were increased in cocultured bTS cells compared with the bTS cells that had been cultured separately (Fig. 4c).
Binucleate and multinucleate bTS cells derivation and gene expression changes in coculture process. a Binucleate and multinucleate bTS cells near/at the border. b The merged staining of KRT18 and DAPI. White arrows indicate binucleate and multinucleate bTS cells. All scale bars represent 100 μm. c The relative quantification of gene expression of bTS cells cocultured with EECs for 12 days. The result shows that the epithelial cell marker KRT18 was decreased, while the mature trophoblast differentiation markers MASH2, GCM1, HAND1 and CDX2 were increased
Discussion
We actually tried to obtain bovine endometrial epithelial cells by different methods such as trypsinization, cutting the uterine wall into small pieces and adherence onto plates. However, EECs derived by trypsinization could not survive by passaging, and the stromal cell contamination was obtained by tissues cutting. Finally, we adopted the method described before (Horn et al. 1998; Skarzynski et al. 2000), but some modifications have been made. Compared with previously published methods (Horn et al. 1998) of EEC derivation, our method simplified the process of digestion by reducing digestion time and adjusted the enzyme solutions from two (enzyme solution I: sterile 0.5 % pancreatin solution containing 4.8 mg/ml dispase II, enzyme solution II: 4.8 mg/ml dispase II in HBSS without Ca2+ and Mg2+) to one enzyme solution (0.5 mg/ml collagenase I and 20 KU/ml deoxyribonuclease I). Notably, after digestion with collagenase I and deoxyribonuclease I for 5 h we did not observe any stromal cell contamination in the bovine EEC culture under a microscope. It is possible that the specific enzymes, the adjusted enzymes concentrations, and the digestion time used in our method resulted in only dissociation of the endomembrane cells on the uterine horn surface without reaching the basal lamina. Furthermore, because stromal cells are more easily dissociated by trypsin treatment than EECs, 0.05 % trypsin was used to eliminate stromal cells during the first passaging. These modifications allowed us to obtain an EEC culture with high purity (>99 %) after the first passaging by flow cytometry analysis and counting KRT18 positive cells. During passaging, the EEC colonies was insensitive to treatment with low concentration of trypsin (≤0.25 %). For example, when the colonies covered more than 80 % of the plate, treatment with 0.25 % trypsin for 10 min failed to get 60 % of the cells into suspension. To avoid affecting EEC activity and and characteristics by digesting the cells with trypsin, we changed our protocol. We first treated the cells with 1 ml of 0.25 % trypsin for 5 min and then scraped the plate with a cell scraper to detach at least 80 % of the cells into suspension for passaging. The obtained EECs maintained the characteristics of epithelial cells for at least 20 passages with no apparent changes in morphology, attachment, or expression of endometrial cell marker genes: IFNAR1, IFNAR2, Erα, PGR, ESR1 and KRT18.
Similar to bovine imEECs, the bovine EECs established in this study expressed both an epithelial marker, cytokeratin, and a stromal marker, vimentin (Zhang et al. 2013). The acquisition of vimentin by cytokeratin-positive epithelial cells during in vitro culture periods has been reported (Bergh et al. 1984; Wang et al. 2000; Zeiler et al. 2007), which has been explained by a limited epithelial to mesenchymal transition (EMT; Pagan et al. 1996). This transition is believed to correlate with a loss of cell-to-cell contacts that result from cell disaggregation during the period of epithelial cell culture preparation (Zeiler et al. 2007). In our study, the vimentin expression may have been acquired during the long deoxyribonuclease I and collagenase I digestion. In vivo, the expression of vimentin in trophoblasts that maintained an epithelial nature has recently been reported (Yamakoshi et al. 2012). However, this was at the trophoblast side, not at the uterine epithelium (Denker 1993).
The establishment of pregnancy requires bidirectional communication between the developing conceptus and the uterine endometrium (Sakurai et al. 2012). An in vitro coculture system with bovine trophoblast cells and uterine epithelial cells can mimic the in vivo attachment process and isolate the essential processes and mechanisms for conceptus attachment to uterine EECs (Munson et al. 1991; Sakurai et al. 2012). One report found that the coculture system resulted in decreased IFNτ and CDX2 mRNA expression levels in CT-1 spheroids, accompanied with changes in histone modifications, as well as an induction of mRNAs for CDX2 and the integrin subunits ITGA8 and ITGB3 in the cocultured CT-1 spheroids (Sakurai et al. 2012). The bTS cells established in our laboratory were similar in phenotype to mouse trophoblast stem cells and had the ability to differentiate into placenta trophoblast cells in vitro (Huang et al. 2014). Ruminants have a distinct population of trophoblastic cells called binucleate cells (BNC) that account for approximately 20 % of the total trophoblast cell population (Wooding et al. 1996). The BNCs are directly involved in modification of the uterine epithelium, beginning at implantation and continuing until term. At maturity, all BNC possess two nuclei and large populations of characteristic granules that produce an array of compounds, including bovine placental lactogens (bPL), prolactin-related protein-1 (bPRP-1) and pregnancy-associated glycoproteins (bPAG; Wooding and Flint 1994). BNCs are also a source of steroid hormones, prostanoids, peptides and other protein hormones. In the present study, when bTS cells were cut into small pieces and cocultured with EECs, the binucleated cells appeared on day 4 of differentiation, and reached 18.47 % on day 12 of coculture, which resembled the in vivo development process, and the expression levels of trophoblast differentiation markers MASH2, HAND1, GCM1 and CDX2 (Huang et al. 2014) were increased in cocultured bTS cells compared with the bTS cells that had been cultured separately (Fig. 4c). However the mechanism underlying the BNC formation needs further investigation.
The development of conceptus and endometrium requires biochemical communications as well as cell-to-cell interactions; therefore, the communication between the conceptus and the uterine epithelium must be studied not only in coculture systems but also in flexible and dynamic cell environments (Krishnaswamy et al. 2009). For these reasons, the bovine EECs established in this study could be a valuable tool for studying such changes, as well as for investigating changes in gene expression, as the implantation process proceeds.
References
Bai H, Sakurai T, Bai R, Yamakoshi S, Aoki E, Kuse M, Okuda K, Imakawa K (2014) Establishment and characterization of immortalized bovine endometrial epithelial cells. Anim Sci J 85:799–804. doi:10.1111/asj.12202
Bergh J, Nilsson K, Dahl D, Andersson L, Virtanen I, Lehto VP (1984) Expression of intermediate filaments in established human lung cancer cell lines. An indicator of differentiation and derivation. Lab Investig J Tech Methods Pathol 51:307–316
Denker HW (1993) Implantation: a cell biological paradox. J Exp Zool 266:541–558. doi:10.1002/jez.1402660606
Fortier MA, Guilbault LA, Grasso F (1988) Specific properties of epithelial and stromal cells from the endometrium of cows. J Reprod Fertil 83:239–248
Hashizume K, Ushizawa K, Patel OV, Kizaki K, Imai K, Yamada O, Nakano H, Takahashi T (2007) Gene expression and maintenance of pregnancy in bovine: roles of trophoblastic binucleate cell-specific molecules. Reprod Fertil Dev 19:79–90. doi:10.1071/Rd06118
Horn S, Bathgate R, Lioutas C, Bracken K, Ivell R (1998) Bovine endometrial epithelial cells as a model system to study oxytocin receptor regulation. Hum Reprod Update 4:605–614
Hou D, Su M, Li X, Li Z, Yun T, Zhao Y, Zhang M, Zhao L, Li R, Yu H, Li X (2015) The efficient derivation of trophoblast cells from porcine in vitro fertilized and parthenogenetic blastocysts and culture with ROCK inhibitor Y-27632. PLoS ONE 10:e0142442. doi:10.1371/journal.pone.0142442
Huang X, Han X, Uyunbilig B, Zhang M, Duo S, Zuo Y, Zhao Y, Yun T, Tai D, Wang C, Li J, Li X, Li R (2014) Establishment of bovine trophoblast stem-like cells from in vitro-produced blastocyst-stage embryos using two inhibitors. Stem Cells Dev 23:1501–1514. doi:10.1089/scd.2013.0329
Krishnaswamy N, Danyod G, Chapdelaine P, Fortier MA (2009) Oxytocin receptor down-regulation is not necessary for reducing oxytocin-induced prostaglandin F(2alpha) accumulation by interferon-tau in a bovine endometrial epithelial cell line. Endocrinology 150:897–905. doi:10.1210/en.2008-0704
Li P, Tong C, Mehrian-Shai R, Jia L, Wu N, Yan Y, Maxson RE, Schulze EN, Song H, Hsieh CL, Pera MF, Ying QL (2008) Germline competent embryonic stem cells derived from rat blastocysts. Cell 135:1299–1310. doi:10.1016/j.cell.2008.12.006
Munson L, Ellington JE, Schlafer DH (1991) Bovine trophoblastic cell vesicle attachment to polarized endometrial epithelial cells in vitro. In Vitro Cell Dev Biol 27:31–38
Pagan R, Martin I, Alonso A, Llobera M, Vilaro S (1996) Vimentin filaments follow the preexisting cytokeratin network during epithelial-mesenchymal transition of cultured neonatal rat hepatocytes. Exp Cell Res 222:333–344
Sakurai T, Bai H, Bai R, Arai M, Iwazawa M, Zhang J, Konno T, Godkin JD, Okuda K, Imakawa K (2012) Coculture system that mimics in vivo attachment processes in bovine trophoblast cells. Biol Reprod 87:60. doi:10.1095/biolreprod.112.100180
Skarzynski DJ, Miyamoto Y, Okuda K (2000) Production of prostaglandin F2α by cultured bovine endometrial cells in response to tumor necrosis factor α: cell type specificity and intracellular mechanisms. Biol Reprod 62:1116–1120
Thie M, Denker HW (2002) In vitro studies on endometrial adhesiveness for trophoblast: cellular dynamics in uterine epithelial cells. Cells Tissues Organs 172:237–252
Wang G, Johnson GA, Spencer TE, Bazer FW (2000) Isolation, immortalization, and initial characterization of uterine cell lines: an in vitro model system for the porcine uterus. In Vitro Cell Dev Biol Anim 36:650–656
Wooding FB, Flint AP (1994) Placentation. In: Lamming GE (ed) Marshall’s physiology of reproduction: volume 3 pregnancy and lactation. Springer, Dordrecht, pp 233–460. doi:10.1007/978-94-011-1286-4_4
Wooding FB, Morgan G, Monaghan S, Hamon M, Heap RB (1996) Functional specialization in the ruminant placenta: evidence for two populations of fetal binucleate cells of different selective synthetic capacity. Placenta 17:75–86
Yamakoshi S, Bai R, Chaen T, Ideta A, Aoyagi Y, Sakurai T, Konno T, Imakawa K (2012) Expression of mesenchymal-related genes by the bovine trophectoderm following conceptus attachment to the endometrial epithelium. Reproduction 143:377–387. doi:10.1530/REP-11-0364
Zeiler M, Leiser R, Johnson GA, Tinneberg HR, Pfarrer C (2007) Development of an in vitro model for bovine placentation: a comparison of the in vivo and in vitro expression of integrins and components of extracellular matrix in bovine placental cells. Cells Tissues Organs 186:229–242. doi:10.1159/000107947
Zhang S, Lin H, Kong S, Wang S, Wang H, Wang H, Armant DR (2013) Physiological and molecular determinants of embryo implantation. Mol Asp Med 34:939–980. doi:10.1016/j.mam.2012.12.011
Acknowledgments
This work was supported by National Transgenic Project of China (2014ZX08010-001) and National Natural Sciences Foundation of China (31460309).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Li, Z., Hou, D. et al. The bovine endometrial epithelial cells promote the differentiation of trophoblast stem-like cells to binucleate trophoblast cells. Cytotechnology 68, 2687–2698 (2016). https://doi.org/10.1007/s10616-016-9994-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-016-9994-x