Abstract
Aesculus hippocastanum (the horse chestnut) seed extract has a wide variety of biochemical and pharmacological effects including anti-inflammatory, antianalgesic, and antipyretic activities. The main active compound of this plant is escin. It is known that several medicinal herbs with anti-inflammatory properties have been found to have a role in the prevention and treatment of cancer. In the present study, the cytotoxic effects of escin in the C6 glioma and A549 cell lines were analyzed by MTT. Apoptotic effects of escin on both cell lines were evaluated by Annexin V binding capacity with flow cytometric analysis. Structural and ultrastructural changes were also evaluated using transmission electron microscopy. The results indicated that escin has potent antiproliferative effects against C6 glioma and A549 cells. These effects are both dose and time dependent. Taken together, escin possesses cell cycle arrest on G0/G1 phase and selective apoptotic activity on A549 cells as indicated by increased Annexin V-binding capacity, bax protein expression, caspase-3 activity and morphological changes obtained from micrographs by transmission electron microscopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer incidence has been experiencing a rise in recent years and is still a major health problem in the world (El Gamal and Oh 2014). Moreover, many of the cancers are highly resistant to chemo and radio-therapy, which induce apoptotic cell death pathways (Heinemann 2002; Cao et al. 2013). Furthermore, most of the chemotherapeutic agents against cancer treatment lead to undesirable side effects (Ciftci et al. 2013; Li et al. 2013). Therefore, new agents, derived from natural products with fewer side effects and the capacity to reduce the high mortality rates of these cancer patients should be investigated. According to ethnobotanical studies, the active compounds of many plants can be used for treatment regimens and participate in modern medicine given their additional therapeutic benefits (Rimmon et al. 2013). Many antitumor agents such as taxanes, vinca alkaloids and anthracyclines are of natural origin and are used in clinical oncology practice indicating a promising future for the use of natural products from plants as anti-tumor agents (Berkovich et al. 2012). One such natural product is the extract of Aesculus hippocastanum (the horse chestnut) seeds. This plant extract has long been known for its antianalgesic, antihemorrhoid and antiedema activities. β-escin or escin, which is the main active compound of this plant seed extract, is a penta cyclic triterpene (Güney and Kutlu 2013) Certainly, escin has a wide variety of biochemical and pharmacological effects. Some studies report that escin has an advantage, which is antagonism to 5-HT and histamine and improved entry of ions into channels, thus raising venous tension. Some of these advantages make escin and its derivatives prime candidates as potential cancer chemotherapeutic agents (Güney and Kutlu 2013). Recent studies show that it also has apoptotic effects in different cancer cell lines (Zhang et al. 2011; Shen et al. 2011).
It also has anti-inflammatory, vasoconstrictor and vasoprotective effects. It has been used for the treatment of chronic venous insufficiency (Sirtori 2001; Pittler and Ernst 2012). It is known that several medicinal herbs with anti-inflammatory properties have been found to have a role in the prevention and treatment of cancer. Therefore, we hypothesized that escin, which is derived from A. hippocastanum seeds and which has anti-inflammatory properties (Rengarajan et al. 2013) could be used as an apoptotic agent against C6 glioma and A549 lung adenocarcinoma. However, few reports focus on the anticancer effects of escin (Zhou et al. 2009; Tan et al. 2010). The mechanisms of escin have never been investigated in C6 glioma cells. Also, the apoptotic effects on A549 cells have never been investigated before. Only one study showed that sodium escin inhibited inducible nitric oxide synthase expression via downregulation of the JAK/STAT pathway in A549 cells (Ji et al. 2011). This means that escin may be a therapeutic agent against inflammatory-associated tumor.
At present, there is dispute over whether escin can reduce the risk of cancer. How escin exhibits its effect on C6 glioma and A549 cell lines and its underlying mechanism are poorly understood. For this reason, in the present study we aimed to evaluate the antiproliferative apoptotic and cell cycle arrest effects of escin in both C6 glioma and A549 human lung adenocarcinoma cell lines.
Materials and methods
Materials
Roswell Park Memorial Institute Medium (RPMI), Dulbecco’s Modified Eagle’s Medium (DMEM), escin, fetal bovine serum (FBS), penicillin, streptomycin, 3-(4.5-dimethyl-2-thiazolyl)-2.5-diphenyl-2 H-tetrazolium (MTT) and dimethylsulfoxide (DMSO) were obtained from Sigma-Aldrich (Seelze, Germany). The Annexin V-FITC [propidium iodide (PI)] apoptosis detection kit and caspase 3 activity kit and cell cycle detection kit (Cycletest Plus DNA Reagent Kit) were purchased from BD Pharmingen (Franklin Lakes, NJ, USA).
Methods
Cell culture and drug treatment
C6 rat glioma cells (ATCC, Rockville, MD, USA) were cultured at 37 °C in a humidified atmosphere of 95 % air and 5 % CO2 in DMEM supplemented with 10 % fetal calf serum. A549 human lung adenocarcinoma cells (ATCC, Rockville, MD, USA) were incubated in 90 % RPMI supplemented by 10 % fetal bovine serum. The stock solutions of escin (Sigma Aldrich, Germany) were prepared in dimethyl sulfoxide (DMSO) and further dilutions were made with fresh culture medium (the concentration of DMSO in the final culture medium was <0.1 %, which had no effect on the cell viability). Exponentially growing cells were plated at 2 × 104 cells/ml into 96-well microtiter tissue culture plates and incubated for 24 h before the addition of the drugs (the optimum cell number for cytotoxicity assays was determined in preliminary experiments). All experiments were performed in duplicate and obtained similar results.
MTT assay for cytotoxicity of escin
The level of cellular reduction of tetrazolium salt, 3-(4.5-dimethylthiazol-2-yl)-2.5-diphenyltetrazolium bromide (MTT) to formazan by mitochondrial succinate dehydrogenase was quantified as previously described in the literature with small modifications. A stock solution of escin (2 mg/ml) in dimethylsulfoxide was prepared and diluted before use. In order to detect the cytotoxicity of escin, C6 and A549 cells were treated with escin to give a final concentration in the range of 1–500 μg/ml for 24 and 48 h. At the end of this period, MTT was added to a final concentration of 0.5 mg/ml and the cells were incubated for a further 4 h at 37 °C. After the medium was removed and the formazan crystals were solubilized by the addition of 200 µL DMSO to each well, the absorbance was read at 540 nm with a microtiter plate spectrophotometer (Bio-Tek plate reader). Each concentration was repeated in three wells and IC50 values were defined as the drug concentrations that reduced absorbance to 50 % of control values.
Flow cytometric analyses of apoptosis
After the cells were incubated with escin at different concentrations (3.5, 7.0, 14.0 or 21.0 μg/ml), phosphotydilserine externalization, which indicates early apoptosis, was measured by Annexin V-PI (BD Pharmingen) on a flow cytometer (BD FACSCalibur™) for 24 h. Annexin V staining protocol was applied according to the manufacturer’s instructions (BD Pharmingen). The cells were then briefly washed with cold PBS and suspended in a binding buffer at a concentration of ~1 × 106 cells/ml. Then, 100 µL of this solution containing ~1 × 105 cells was transferred to a 5 ml test tube. After 5 µL of Annexin-V and PI was added, the cells were incubated for 15 min at room temperature (RT) in the dark. Then 400 µL of 1× Binding Buffer was added to each tube and the cells were processed for data acquisition, and analyzed on a Becton–Dickinson FACS Aria using FACSDIVA Version 6.1.1. Software.
Flow cytometric analyses of Caspase 3
After A549 cells were incubated with escin at 3.5, 7, 14 and 21 μg/ml concentrations for 24 h, Caspase 3 activity measurement protocol was applied according to the manufacturer’s instructions (BD, Biosciences Pharmingen). The cells were briefly washed with cold PBS and incubated with 0.5 mL perm lyse solution for 30 minutes at room temperature in the dark. Pellets were washed twice with 0.5 ml perm wash buffer. Cells were resuspended in 100 ml perm wash buffer and 10 ml Caspase 3 antibody was added for 20 min at RT in the dark. At least 10,000 cells were counted for each sample and the cells were analyzed by flow cytometry (BD FACSCalibur™) using FACS Diva Version 6.1.1. Software.
Transmission electron microscopy (TEM)
TEM (TEM FEI Tecnai BioTWIN; Hillboro, OR, USA) was processed for ultrastructral changes. C6 and A549 cells growing in DMEM and RPMI, respectively, were incubated with 11.5, 23, 34.5 and 7, 14, 21 μg/ml of escin, respectively, for 24 h. They were then fixed with 2.5 % glutaraldehyde in 0.1 M phosphate buffer (PBS) at pH 7.4 and left in P BS overnight at +4 °C. After being embedded in agar and post fixation in 2 % osmium tetroxide, cells were dehydrated in graded ethanol: 70, 90, 96 and 100 %. Then cells were embedded in EPON 812 epoxy (Sigma Aldrich, Seelze, Germany) and sectioned on ultramicrotome (LEICA EM UC6; Wetzlar, Germany).
Cell cycle analysis
After A549 cells were incubated with escin at 3.5, 7, 14 and 21 μg/ml concentrations for 24 h cell cycle analyses measurement protocol was applied according to the manufacturer’s instructions (BD, Biosciences). Then the cells were briefly suspended in the citrate buffer. The cells were then centrifuged at 400 g for 5 min at RT. The supernatant was decanted and 250 μl of solution A was added to the pellet and kept at RT for 10 min. Then, 200 μl of solution B was added, gently mixed and kept at RT for 10 min. Then 200 μl of solution C was added. After being gently mixed, it was kept in the dark at 4 °C for 10 min and then analyzed on a flow cytometer using BD Bioscience’s MODFID software.
Immunohistochemical analysis of bax
A549 cells which were incubated with escin (14 and 21 μg/ml) for 24 h, were fixed in 3.7 % formaldehyde solution for 30 min. Then cells were collected in tubes and centrifuged at 1200 rpm for 5 min. Cells were carried to slides. Slides were incubated in methanol for 10 min at −20 °C. Then heat-induced antigen retrieval was carried out. The antigen retrieval buffer (10 mM sodium citrate pH 0.6) was boiled and powered off, and put on the slides. After retrieving the heat-induced antigen, the slides were allowed to cool and then rinsed in distilled water. Endogenous peroxidase was inhibited by incubating the slides with 3 % hydrogen peroxidase for 5 min at room temperature. Specific epitope binding was then blocked by incubation for 40 min with 20 % goat serum (ab-7481; Abcam, Cambridge, U.K). Slides were washed with phosphate buffer solution (PBS, pH 7.4) for three times. Then, slides were incubated at 4 °C for 1 h with dilutions of primary antibodies against Bax (Abcam, CatNo ab-7977) (dilution 1:200). An isotype control was also employed to identify non-specific binding from the HRP polmer secondary antibody (ab-2891, Abcam). Slides were washed with (PBS, pH 7,4) for three times. The samples were subsequently incubated with the secondary antibodies for 30 min. Slides were then visualized using chromogen (AEC) for 5 min and counterstained with hematoxylin for 1 min. The sections were washed in PBS, dried, mounted in aqueous mounting medium and observed at 400× magnification. Brown particles were considered as positive area; protein expressions in different groups were evaluated.
Statistical analyses
Statistical analyses were evaluated using Statistical Package for Social Science (SPSS) for Windows 15.0. Data were expressed as Mean ± SD. Comparisons were performed by one way ANOVA test for normally distributed continuous variables and Tukey test was used for post hoc analyses of group differences. p < 0.05 was considered as statistically significant.
The apoptotic re sults (Annexin V-FITC and Caspase 3) were evaluated by flow cytometer using FACS Diva Version 6.1.1. Software, and the apoptotic cells were determined as the percentage of cells. Cell cycle analyses were evaluated by flow cytometer using BD Bioscience’s MODFID software.
Results and discussion
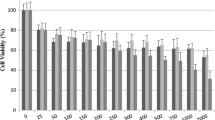
In the present study, the cytotoxic and apoptotic effects of escin were investigated in C6 glioma and A549 lung adenocarcinoma cells using in vitro MTT, Annexin V–FITC, Caspase-3, bax protein expression and transmission electron microscopy (TEM) test systems. We examined the effects of different concentrations of escin on the viability of C6 glioma and A549 cell lines for 24 and 48 h by the MTT method (Tables 1, 2). Escin inhibited C6 and A549 cell viabilities in a dose and time dependent manner. (Tables 1, 2). IC 50 values were determined in C6 cells as 23 and 16.3 μg/ml for 24 and 48 h, respectively (Table 1). Also, IC50 values were determined in A549 cells as 14 and 11.3 μg/ml for 24 and 48 h, respectively. The viability of cells was decreased when the dose of escin was increased (Fig. 1).
Flow cytometric analysis of C6 glioma cells treated with different concentrations of escin for 24 h. a, b Control, c 5.75 μg/ml escin treated cells, d 11.5 μg/ml escin treated cells, e 23 μg/ml escin treated cells, f 34.5 μg/ml escin treated cells. At least 10,000 cells were analysed per sample, quadrant analysis was performed. Each quadrant shows the proportion (%) of cell number. Q1 necrotic cells; Q2 late apoptotic cells; Q3 viable cells; Q4 early apoptotic cells (b–f). a shows the total cell population analysed by flow cytometer
Flow cytometric analysis of A549 human lung adenocarcinoma cells treated with different concentrations of escin for 24 h. a, b Control, c 3.5 μg/ml escin treated cells, d 7 μg/ml escin treated cells, e 14 μg/ml escin treated cells, f 21 μg/ml escin treated cells. At least 10,000 cells were analysed per sample, quadrant analysis was performed. Each quadrant shows the proportion (%) of cell number. Q1 necrotic cells; Q2 late apoptotic cells; Q3 viable cells; Q4 early apoptotic cells (b–f). a shows the total cell population analysed by flow cytometer
Morphological changes of C6 glioma cells treated with different concentrations of escin for 24 h. a Control (scale: 2 μm); b 11.5 μg/ml escin treated cells (scale: 2 μm); c 23 μg/ml escin treated cells (scale: 1 μm); d 23 μg/ml escin treated cells (scale: 500 nm); N Nucleus, L Lysosome; M Mitochondria. White arrows show lysosomes. Red arrows show DNA condensation. Green arrow shows mitochondrial damage. Blue arrow shows damage of membranes. c, d show cellular morphological changes in same cells (DNA condensation was observed and mitochondria and membranes of organels were damaged on 23 μg/ml escin treated C6 glioma cells)
In this study it was shown that A549 cells were more sensitive to escin than C6 glioma cells and underwent rapid apoptosis on treatment with escin, as indicated by increased Annexin V-binding capacity with flow cytometric analysis. When A549 cells were incubated with different concentrations of escin (3.5, 7, 14, 21 μg/ml), early apoptotic cell percentages were determined as 1.6, 6.0, 26.2 and 31.6 (Fig. 2; Table 4). Furthermore, late apoptotic cell percentages were determined as 2.4, 4.6, 7.1 and 32.2 on the incubations with different concentrations of escin (Fig. 2; Table 4). These results show that increasing concentrations of escin on A549 cells induced apoptosis, which involves DNA condensation, fragmentation and mitochondrial changes (Dietze et al. 2001). These results were consistent with TEM micrographs. At increasing concentrations of escin, the shape of the treated cells became rounded, the cellular organization was disrupted, cell shrinkage, DNA condensation were observed and membranes of organels were sharply damaged for both 14 and 21 μg/ml escin treated cells, indicating morphological changes of apoptosis (Fig. 4c, d). The large vacuoles in the cytoplasm containing degraded cellular material were increased and membranes were slightly damaged in the incubations with 7 μg/ml concentration of escin (Figs. 3, 4b).
Morphological changes of A549 human lung adenocarcinoma cells treated with different concentrations of escin for 24 h. a Control (scale: 2 μm); b 7 μg/ml escin treated cells (scale: 2 μm); c 14 μg/ml escin treated cells (scale: 5 μm); d 14 μg/ml escin treated cells (scale: 2 μm); N Nucleus. Yellow arrows show vacuoles in cells. Blue arrow shows damage of membranes. Red arrows show DNA condensation. c, d show cellular morphological changes in same cells (The shape of the treated cells became round, the cellular organization was disrupted, cell shrinkage and DNA condensation were observed and membranes of organels were sharply damaged)
On the other hand, C6 cells were incubated with different concentrations of escin for flow cytometric analyses. An escin concentration of 23 μg/ml (IC50) caused 10.5 % apoptotic cells. When the concentration was increased to 34.5 μg/ml, apoptotic cell percentage decreased (7.4 %) (Fig. 1; Table 3). These results were consistent with TEM micrographs shown in Fig. 3d. Furthermore, DNA condensation was observed and mitochondria and membranes of organels were damaged on 23 μg/ml escin treated C6 glioma cells indicating morphological changes due to apoptosis (Fig. 3c). The lysosomes of cells were increased and morphology was slightly changed on incubation with at a concentration of escin of 11.5 μg/ml (Fig 3b).
Different previous studies with different cell lines have obtained results supporting our findings. Güney and Kutlu (2013) reported that escin inhibited growth of H-Ras 5RP7 cell line, induced apoptosis and caspase-3 dependent activity. Also, TEM micrographs confirmed that escin damaged these cells and induced apoptosis. These results were obtained in a dose and time-dependent manner as seen in our study (Güney and Kutlu 2013). Different cancer cell lines exhibit differential sensitivity to escin, suggesting that the escin effects vary based on the cell type. Shen et al. (2011) indicated that escin induced apoptosis of human cholangiocarcinoma cell lines through mitochondrial caspase-dependent pathway. In that study different methods and flow cytometry were used and apoptotic results were obtained similar to ours. In addition to inhibition of cell growth and inducing apoptosis, many mechanisms have been proposed to explain the antitumoral effect of escin, including its anti-inflammatory and antioxidant activities (Ji et al. 2011; Wang et al. 2001). In some in vitro studies escin showed anti-apoptotic effects (Dietze et al. 2001). Wang et al. (2001) showed that escin can protect from lung injury induced by intestinal ischemia/reperfusion (I/R). In this study escin inhibited lipid peroxidation, upregulated Bcl-2 gene protein expression, and improved the ratio of Bcl-2/Bax to inhibit lung apoptosis. Hu et al. (2001) obtained results similar to Wang et al. (2001). They showed that beta-escin inhibited caspase-3 activation and the release of cytochrome c increased the expression of Bcl-2 after cerebral I/R in rats. Also, escin has shown synergistic effects with 5- fluorouracil in human hepatocellular carcinoma SMMC-7721 cells (Ming et al. 2011). Escin is described as an efficient apoptotic agent in various cell lines. However, its mechanism varies depending on cell lines. In addition to these data concentration of escin used for inducing apoptosis varies in different studies. Güney and Kutlu (2013) showed that escin caused growth inhibition of 5RP7 cells with a half maximal inhibitory concentration (IC50) value of 35.95 ± 0.35 μM. In our study, IC50 value was determined as 23 μg/ml for C6 glioma (Table 1) and 14 μg/ml for A549 cell lines (Table 2). According to these results A549 cells were more sensitive for escin than C6 glioma cells (Table 4).
Furthermore, we studied the caspase-3 activity, immunohistochemical bax protein expression by histochemistry and cell cycle analyses of escin on A549 cells to enlighten action on apoptosis. Escin induced caspase-3 activity in a dose-dependent manner on A549 cells (Table 5; Fig 5). Caspases, which are activated to trigger apoptosis, affect cell cycle regulation and signaling pathways together with the morphological manifestations of apoptosis, such as DNA condensation and fragmentation, and membrane blebbing (Mancini et al. 1998). Caspase-3 activation was observed by escin on different cell lines (Güney and Kutlu 2013; Shen et al. 2011). Shen et al. 2011 showed that the apoptotic effect of escin was associated with the collapse of the mitochondrial membrane potential and activation of caspase-3 in cholangiocarcinoma cells. Zhang et al. (2011) showed that escin activated caspase-3 in a selective manner in human acute leukemia Jurkat T cells. In our study, both 14 and 21 μg/ml concentrations of escin also induced bax protein expression of A549 cells compared to control cells. Cisplatin was used as positive control. In our previous study, the IC50 value for cisplatin was determined as 19 μg/ml (Yurttaş et al. 2013). This result showed that escin used the mitochondrial pathway for inducing apoptotic cell death (Figs. 6, 7a, b, c).
Flow cytometric analysis of caspase-3 activity of A549 human lung carcinoma cells treated with different concentrations of escin for 24 h. a, b Control, c 3.5 μg/ml escin treated cells, d 7 μg/ml escin treated cells, e 14 μg/ml escin treated cells, f 21 μg/ml escin treated cells. At least 10,000 cells were analysed per sample, quadrant analysis was performed (b-f). a shows the total cell population analysed by flow cytometer
Cell cycle distribution of A549 cells treated with different concentration of escin for 24 h. a, b Control c 3.5 μg/ml escin treated cells, d 7 μg/ml escin treated cells, e 14 μg/ml escin treated cells, f 21 μg/ml escin treated cells. At least 10,000 cells were analysed per sample. a shows the cell population analysed by flow cytometer. Blue color shows debris, green color shows aggregates and red color shows cells in G1,G2 phases
Bax protein expression of A549 human lung adenocarcinoma cells treated with different concentrations of escin for 24 h (immunohistochemical analysis). a Control, b 14 μg/ml escin treated cells, c 21 μg/ml escin treated cells, d 19 μg/ml cisplatin treated cells. Blue color shows nucleus and brown particles show positive area for bax protein
The progression of cell proliferation encounters three major checkpoints including G1/S, S and G2/M (Ming et al. 2011). Escin has been shown to induce arrest of the cell cycle in cancer cell lines. Ming et al. (2011) showed that 21.6 μg/ml of escin caused a 4.9 % increase at G0/G1 cell cycle arrest in human hepatocellular carcinoma cells compared to controls. However, we observed more elevated G0/G1 cell cycle arrest (31.76 %) in A549 cells compared to the controls at a concentration of 21 μg/ml (Table 6; Fig. 6). Furthermore, G0/G1 cell cycle arrest was dose dependent (3.16, 7.36 and 23.76 % for 3.5, 7, 14 μg/ml of escin, respectively) (Table 6; Fig 6). S hen et al. (2011) showed that escin caused an arrest in the G2/M phase in two cholangiocarcinoma cells (QBC939, and Sk-ChA-1). These apparent discrepancies may result from differences in cell lines. This result suggests that the inhibitor effect of escin on proliferation was related to a cell cycle regulation effect and that escin may induce apoptosis of A549 cells in the G0/G1 phase of the cell cycle (Takahaski et al. 2014). Since apoptosis is almost always found in proliferating cells, molecules acting on cells in the late G1 phase are also required for apoptosis induction. The correlation between cell cycle and apoptosis was shown by Kim et al. (2001). They showed that caspase-3 cleaves RB, p21 and p27 via induction of cell cycle arrest in cells by tumor growth factor (TGF)-β1 administration and caspase-3 activation by TGF-β1 may initiate the conversion of cell-cycle arrest to apoptosis (Alenzi 2004).
In conclusion, escin has potent antiproliferative effects against C6 glioma and A549 cells. These effects are dose and time dependent. Also, escin caused apoptotic effects which were more intensely observed in A549 cells compared to C6 glioma. The most likely mechanisms of action are the arrest of cell cycle and the induction of apoptosis by caspase-3 activation and bax protein expression on A549 cells. This study suggests that escin may be a suitable candidate for tumor treatment. Although escin induces apoptosis of A549 cells by mitochondrial death signaling pathway, additional experiments are necessary to explain any additional mechanisms of action for escin on C6 cell lines.
References
Alenzi FQ (2004) Links between apoptosis, proliferation and cell cycle. Br J Biomed Sci 61:99–102
Berkovich L, Ron I, Earon G, Abu-Ghanem S, Rimmon A, Lev-Ari S (2012) The role of medicinal herbs with anti-inflammatory properties in prevention and treatment of cancer. Harefuah 151:629–632
Cao H, LE D, Yang LX (2013) Current status in chemotherapy for advanced pancreatic adenocarcinoma. Anticancer Res 33:1785–1791
Ciftci GA, Temel HE, Yıldırım SU, Kaplancıklı ZA, Altıntop MD, Genc L (2013) Apoptotic effects of some carbazole derivatives on lung carcinoma and glioma cell lines. Med Chem Res 22:3751–3759
Dietze EC, Caldwell LE, Grupin SL, Mancini M, Seewaldt VL (2001) Tamoxifen but not 4-hydroxytamoxifen initiates apoptosis in p53(-) normal human mammary epithelial cells by inducing mitochondrial depolarization. J Biol Chem 276:5384–5394
El-Gamal MI, Oh CH (2014) Diarylureas and diarylamides with pyrrolo[2,3-d]pyrimidine scaffold as broad-spectrum anticancer agents. Chem Pharm Bull 62:25–34
Güney G, Kutlu HM (2013) The apoptotic effects of escin in H-Ras transformed 5RP7 cell line. Phytother Res 27:900–905. doi:10.1002/ptr.480
Heinemann V (2002) Present and future treatment of pancreatic cancer. Semin Oncol 29:23–31
Hu XM, Zhang Y, Zeng FD (2001) Effects of beta-aescin on apoptosis induced by transient focal cerebral ischemia in rats. Phytomedicine 17:575–580
Ji DB, Xu B, Liu JT, Ran FX, Cui JR (2011) Escin sodium inhibits inducible nitric oxide synthase expression via downregulation of the JAK/STAT pathway in A549 cells. Mol Carcinog 50:945–960
Kim SG, Kim SN, Jong HS, Kim NK, Hong SH, Kim SJ, Bang YJ (2001) Caspase mediated Cdk2 activation is a critical step to execute transforming growth factor-beta1-induced apoptosis in human gastric cancer cells. Oncogene 20:1254–1265
Li YX, Himaya SW, Dewapriya P, Zhang C, Kim SK (2013) Fumigaclavine C from a marine derived fungus Aspergillus fumitas induces apoptosis in mcf-7 breast cancer cells. Mar Drugs 13:5063–5086. doi:10.3390/md11125063
Mancini M, Nicholson DW, Roy S (1998) The caspase-3 precursor has a cytosolic and mitochondrial distribution: implications for apoptotic signaling. J Cell Biol 140:1485–1490
Ming ZJ, Hu Y, Qiu YH, Cao L, Zhang XG (2011) Synergistic effects of beta-aescin and 5-fluorouracil in human hepatocellular carcinoma SMMC -7721 cells. Mol Carcinog 50:945–960
Pittler MH, Ernst E (2012) Horse chestnut seed extract for chronic venous insufficiency. Cochrone Database Syt Rev 14(11):CD003230. doi:10.1002/14651858.CD003230.pub4
Rengarajan T, Rajendran P, Nandakumar N, Balasubramanian MP, Nishigaki I (2013) Cancer preventive efficacy of marine carotenoid fucothin: cell cycle arrest and apoptosis. Nutrients 6:4978–4989
Rimmon A, Vexler A, Berkovich L, Earon G, Ron I, Lev-Ari S (2013) Escin chemosensitizes human pancreatic cancer cells and inhibits the nuclear factor-kappaB signaling pathway. Biochem Res Int 2013:251752. doi:10.1155/2013/251752
Shen DY, Kang JH, Song W, Zhang WQ, Li WG, Zhao Y, Chen QX (2011) Apoptosis of human cholangiocarcinoma cell lines induced by b-escin through mitochondrial caspase-dependent pathway. Phytother Res 25:1519–1926
Sirtori CR (2001) Aescin: pharmacology, pharmacokinetics and therapeutic profile. Pharmacol Res 44:183–193
Takahaski A, Kimura F, Yamanaka A, Takebayashi A, Kita N, Takahaski K, Murakami T (2014) Metformin impairs growth of endometrial cancer cells via cell cycle arrest and concomitant autophagy and apoptosis. Cancer Cell Int 14:53
Tan SM, Li F, Rajendran P, Kumar AP, Hui KM, Sethi G (2010) Identification of beta-escin as a novel inhibitor of signal transducer and activator of transcription3/Janus-activated kinase 2 signaling pathway that suppresses proliferation and induces apoptosis in human hepatocellular carcinoma cells. J Pharmacol Exp Ther 334:285–293
Wang YL, Jing YL, Cai QY, Cui GJ, Zhang YB, Zhang FY (2001) Effects of sodium aescinate on the apoptosis-related genes in lung injury induced by intestinal ischemia reperfusion in rats. Acta Pharmacol Sin 25:1267–1275
Yurttaş L, Demirayak Ş, Çiftçi GA, Yıldırım ŞU, Kaplancıklı ZA (2013) Synthesis and biological evaluation of some 1,2- disubstituted benzimidazole derivatives as new potential anticancer agents. Arch Pharm 346:403–414
Zhang Z, Gao J, Cai X, Zhao Y, Wang Y, Lu W, Gu Z, Zhang S, Cao P (2011) Escin sodium induces apoptosis of human acute leukemia Jurkat T cells. Phytother Res 25:1747–1755
Zhou XY, Fu FH, Li Z, Dong QJ, He J, Wang CH (2009) Escin, a natural mixture of triterpene saponins, exhibits antitumor activity against hepatocellular carcinoma. Planta Med 75:1580–1585
Acknowledgments
This work was carried out in the Anadolu University Medicinal Plants, Drugs, and Scientific Research Center.
Conflict of interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Çiftçi, G.A., Işcan, A. & Kutlu, M. Escin reduces cell proliferation and induces apoptosis on glioma and lung adenocarcinoma cell lines. Cytotechnology 67, 893–904 (2015). https://doi.org/10.1007/s10616-015-9877-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-015-9877-6