Abstract
Diabetes is one of the most prevalent chronic diseases globally. In this study, major polyphenols (17.35 ± 0.93–36.66 ± 2.01 mg/g) and minor fucoxanthin (non detected 15.12 ± 0.09 mg/g) were isolated from water, ethanol, and acetone extracts (WES, EES, and AES, respectively) of Sargassum hemiphyllum. Inhibition of α-amylase, α-glucosidase, sucrose, and maltase activities and stimulation of insulin secretion was greater with AES than with WES or EES and correlated with polyphenol and fucoxanthin concentrations in extracts. Moreover, 250 μg/ml EES and AES significantly increased insulin secretion in the presence of 25 mg/ml glibenclamide to higher levels than those obtained with 50 mg/ml glibenclamide. None of the extracts exhibited cytotoxicity, exacerbated the side effects of glibenclamide, or inhibited glibenclamide-induced insulin secretion. These results suggested that the S. hemiphyllum extracts WES, EES, and AES could be used as pharmaceuticals and functional foods to reduce dosages of synthetic diabetes drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The international diabetes federation (IDF) estimated that 285 million people, corresponding to 6.4 % of the world’s adult population, lived with diabetes in 2010 and that this number is expected to grow to 438 million by 2030. Diabetes is one of the most serious and chronic diseases globally, and its incidence and severity increase with obesity and aging (IDF Diabetes Atlas 2009). Type II diabetes accounts for more than 90 % of all diabetes cases (Zimmet et al. 2001), and unlike type I diabetes, it is not insulin dependent and cannot be effectively controlled by administration of insulin.
Hyperglycemia, a condition characterized by abnormal postprandial increase in blood glucose levels, has an important role in patients with type II diabetes. It appears that long term exposure to high glucose concentrations causes toxicity to pancreatic β-cells, leading to defective β-cell gene expression, insulin synthesis, and impaired glucose-stimulated insulin secretion (Roberston et al. 1992). Moreover, hyperglycemia reduces insulin secretion in type II diabetes (Weir et al. 2001). Thus, maintenance of blood glucose concentrations within normal ranges in type II diabetes patients is accomplished using oral anti-hyperglycemic and insulin stimulating agents. Currently, anti-diabetes α-glucosidase inhibitors, such as acarbose, slow the digestion of carbohydrates from food and help to lower blood glucose concentrations after meals (Jenkins et al. 1981). Other diabetes medications, such as glibenclamide, are known as sulfonylureas that inhibit ATP-sensitive potassium channels in pancreatic β-cells, resulting in increased insulin secretion (Serrano-Martín et al. 2006). However, these synthetic drugs cause side effects such as abdominal distention, flatulence, meteorism, hypoglycemia, and cholestasis (Monami et al. 2006). Therefore, natural substances that enhance the efficacy of such drugs without side effects are currently required. Recently, there has been great interest in finding lead compounds from marine sources. In particular, brown seaweeds contain numerous biologically potent chemicals, and some of these have anti-diabetic activities. For example, extracts of Ascophyllum nodosum (Apostolidis and Lee 2010; Zhang et al. 2007), Ecklonia stolonifera (Iwai 2008), Ishige okamurae (Heo et al. 2009), and Spatoglossum schroederi (Teixeira et al. 2007) strongly inhibited α-amylase and/or α-glucosidase activities. Moreover, extracts of Undaria pinnatifida significantly lowered insulin resistance indices (Park et al. 2011), and seaweed polysaccharide compounds were shown to stimulate insulin secretion (Zhang et al. 2008). However, currently there are no reports of brown seaweed extracts that achieve both effects. Thus, the objective of this study was to examine the effects of the brown seaweed Sargassum hemiphyllum, a traditional Chinese medicine in Taiwan (Yao 2003), on α-amylase and α-glucosidase activities and the subsequent insulinogenic effects.
Materials and methods
Preparation of S. hemiphyllum extracts
Fresh S. hemiphyllum was collected from the coast of Penghu county, Taiwan, from December 2009 to January 2010. S. hemiphyllum specimens were washed and dried in current air (RISEN Co., Taipei, Taiwan) at 40 °C for 90 min. Dried samples were ground to flour using a mini blender (YOUQI, Taipei, Taiwan) and were then dried (RISEN Co., Taipei, Taiwan) at 50 °C for 10 min. Hundred grams of dried S. hemiphyllum was boiled in 5 l of distilled water at 100 °C for 30 min. The extract was centrifuged at 10,000×g for 20 min; the supernatant was lyophilized under reduced pressure (2 mmHg) at −20 °C and 10.30 g of S. hemiphyllum water extract (WES) was obtained. S. hemiphyllum samples of 100 g were shaken in 2 l of 95 % ethanol or 70 % acetone at 70 °C for 6 h. Ethanol and acetone extracts were centrifuged at 10,000×g for 20 min, and supernatants were evaporated under reduced pressure at <40 °C. Subsequently, 2.66 g of S. hemiphyllum ethanol extract (EES) and 2.13 g of S. hemiphyllum acetone extract (AES) were obtained. S. hemiphyllum extracts (WES, EES, and AES) were stored at −20 °C prior to use in assays.
Polyphenol assay
The polyphenol concentration was determined using the method of the International Organization for Standardization (ISO; 14502-1, 2005). In brief, 1.0 ml of aliquots of diluted extracts were transferred in duplicate to separate tubes containing 5.0 ml of a 1/10 dilution of Folin–Ciocalteu reagent in water. Subsequently, 4.0 ml of sodium carbonate solution (7.5 % w/v) was added and the tubes were allowed to stand at room temperature for 60 min before measuring absorbance at 765 nm using water as a blank and gallic acid as a standard.
Purification and fucoxanthin assay
The fucoxanthin concentration was determined in extracts using HPLC analyses after purification. Purification was performed using the method of Stauber and Jeffrey (1988) with minor modifications. Extracts were dissolved in 95 % ethanol and were applied to a cartridge column (Column PD-10, GE, Piscataway, NJ, USA) with silica gel (Keisel gel 60, Merck, Darmstadt, Germany). The column was equilibrated with hexane, and stepwise elution was performed with hexane:ethyl acetate mixtures of 10:0–4:6 v/v. Fuoxanthin was recovered in the 5:5–4:6 hexane:ethyl acetate fraction. Reversed-phase HPLC was conducted using a Hitachi L-7000 system on a Develosil ODS-UG-5 (250 × 4.6 mm i.d., 5.0 μm particle size; Nomura Chemical Co., Aichi, Japan) fitted with a guard column (10 × 4.0 mm i.d.) containing an identical stationary phase. A 70:30 (v/v) mixture of methanol and acetonitrile was used as the mobile phase at a flow rate of 1.0 ml/min. Fucoxanthin was monitored at 450 nm using a UV–Vis detector (Kotake-Nara et al. 2001).
Inhibitory activity assay of α-amylase and α-glucosidase (sucrase and maltase)
Inhibition of α-amylase, sucrose, and maltase was determined according to the modified method of Kato et al. (2008) using α-amylase from porcine pancreas (Sigma Chem., St. Louis, MO, USA) and starch as the substrate. The remaining starch was determined colorimetrically using an Amylase test from Wako (Wako Pure Chemical Ind., Osaka, Japan). Subsequently, a crude enzyme solution of α-glucosidase, sucrose, and maltase was prepared from rat small intestinal acetone powder (Sigma Chem., St. Louis, MO, USA). The powder was homogenized in a nine fold volume of ice cold 56 mM maleate buffer (4 °C, pH 6.0), centrifuged at 2,555×g for 10 min, and the supernatant was used as assays. The activities of sucrase and maltase were determined using sucrose and maltose as substrates, respectively. Released d-glucose was determined colorimetrically using a Glucose CII test from Wako (Wako Pure Chemical Ind., Osaka, Japan) and acarbose as a positive control. Inhibitory activities of extracts were expressed as a percentage of untreated enzyme activity. Median inhibitory concentrations (IC50) of extracts were calculated using linear regression curves of extract concentrations versus percent enzyme activity.
Cell culture
RIN-5F rat β-cells were obtained from the American Type Culture Collection (Manassas, VA, USA). RIN-5F cells were cultured in RPMI 1640 medium (Sigma, Poole, UK) supplemented with 2 mM glutamine, 0.5 units/ml penicillin, 500 ng/ml streptomycin, and 10 % (v/v) fetal bovine serum (Invitrogen Gibco BRL, Grand Island, NY, USA) at 37 °C in an incubator containing 5 % CO2. The medium was renewed every 2 days.
Cell culture in different glucose concentration
RIN-5F cells were seeded into 24-well plates at 2 × 105 cells/well. Two days after plating, when most cells were attached and began to flatten, cells were cultured for 10 days in medium containing 5.6, 11, or 28 mM glucose. Glucose enriched medium was renewed every 2 days. Subsequently, cells were incubated in fresh culture medium containing 1 mg/ml 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) for 4 h, and absorbance was recorded at 570 nm using an enzyme-linked immunosorbent assay (ELISA) reader (Molecular Devices, Tokyo, Japan; Mosmann 1983). Finally, cell culture supernatants were collected and assayed for insulin secretion.
Cell viability assay
RIN-5F cells (2 × 105 cells/well) were cultured in RPMI 1640 medium containing 28 mM glucose for 10 days. Medium was then replaced with fresh medium containing 10 μl of 10, 100, and 250 μg/ml extracts, and cells were cultured for a further 24 h. Cell viability was determined using the MTT colorimetric assay (Vittimberga et al. 1999). In brief, cells were treated with MTT (1 mg/ml) for 4 h and absorbance was recorded at 570 nm (Mosmann 1983). Percent cell viability was determined as (A1/A0) × 100 %, where A1 and A0 are absorbance in the presence and absence of extracts, respectively.
Sargassum hemiphyllum extracts loading test treatment
RIN-5F cells (2 × 105 cells/well) were cultured in RPMI 1640 medium containing 28 mM glucose for 10 days. Subsequently, cells were washed in fresh RPMI 1640 medium to remove released insulin.
Sargassum hemiphyllum extracts (WES, EES and AES)
RIN-5F cells were cultured with or without 10 μl of 20 mg/ml glucose solutions for 5 min, and 10 μl of 10, 100 and 250 μg/ml S. hemiphyllum extracts, or 10 μl of 50 mg/ml glibenclamide solution (Sigma Chem., St. Louis, MO, USA) were added. After 3 h of incubation, cell culture supernatants were collected and assayed for insulin secretion.
The combination of S. hemiphyllum extracts and glibenclamide
RIN-5F cells were treated with 10 μl of 250 μg/ml extracts with or without 10 μl of 25 mg/ml glibenclamide. After 3 h of incubation, cell culture supernatants were collected and assayed for insulin secretion.
Combination treatments with amylin and S. hemiphyllum extracts or glibenclamide
RIN-5F cells were treated with 10 μl of 250 μg/ml extracts or 50 mg/ml glibenclamide with or without 10 μl of 0.3 mg/ml amylin (Sigma Chem., St. Louis, MO, USA). After 3 h of incubation, cell culture supernatants were collected and assayed for insulin secretion.
Insulin secretion assay
Insulin concentrations were determined in supernatants using an ELISA kit for human insulin (Mercodia, Uppsala, Sweden).
Statistical analysis
All experimental data were analyzed using one-way ANOVA. Differences are reported as significant when p < 0.05.
Results and discussion
Polyphenol and fucoxanthin contents of S. hemiphyllum extracts
Seaweeds are exposed to strong light and high oxygen concentrations, but avoid oxidative damage to structural components through the action of abundant antioxidants (Wong et al. 1999). Extraction of various antioxidants from seaweeds require solvents of varying polarity. Similarly, antioxidant yields have a critical effect on the polarity of solvents (Waterman and Mole 1994). Thus, for antioxidant extraction from S. hemiphyllum, we used water, 95 % ethanol, and 70 % acetone as solvents in order of decreasing polarity. Initial screening of the three extracts showed that AES had the highest polyphenol concentration (36.66 ± 2.01 mg/g), followed by EES (22.35 ± 1.41 mg/g) and WES (17.35 ± 0.93 mg/g). Acetone may increase the total yield by inhibiting interactions between proteins and polyphenols during extraction (Hagerman 1988) or even by breaking hydrogen bonds (Porter 1989). Interestingly, fucoxanthin, which significantly lowers blood glucose concentrations and plasma insulin levels (Maeda et al. 2008), was also detected in EES (7.89 ± 0.03 mg/g) and AES (15.12 ± 0.09 mg/g) but not in WES (Table 1).
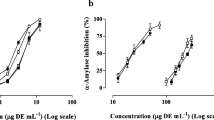
Inhibition of α-amylase and α-glucosidase, sucrose, and maltase activities by S. hemiphyllum extracts
The carbohydrate metabolism enzyme α-amylase hydrolyzes α-1, 4-glucosidic linkages of starch, glycogen and various other oligosaccharides. Subsequently, α-glucosidase metabolizes disaccharides into simple sugars for intestinal absorption. Thus, inhibition of these enzymes controls diabetes by diminishing the absorption of glucose (Hara and Honda 1990). In the present study, IC50 values of AES for α-amylase, sucrose, and maltase were 0.35 ± 0.05, 1.89 ± 0.03, and 0.09 ± 0.01 mg/ml, respectively. In contrast, EES only inhibited sucrase and maltase, with IC50 values of 3.47 ± 0.10 and 2.88 ± 0.09 mg/ml, and WES failed to inhibit α-amylase, sucrose, or maltase (Table 1). The IC50 values of acarbose for α-amylase, sucrose, and maltase were 0.70 ± 0.05, 0.39 ± 0.02, and 0.35 ± 0.07 μg/ml (data not shown). According to these data, AES, EES, and WES had decreasing enzyme inhibitory activities and polyphenol and fucoxanthin concentrations in this order. Moreover, a previous report also indicated that in the extracts, the concentrations of polyphenol compounds and the inhibitory effect to α-glucosidase were positively correlated (Apostolidis and Lee 2010; Iwai 2008). Adefegha and Oboh (2012) reported that the inhibitory potentials of polyphenol-rich extracts on α-amylase and α-glucosidase corresponded with antioxidant properties. Thus, we suggest that α-amylase, sucrose, and maltase inhibitory activities are also correlated with polyphenol and fucoxanthin concentrations, which were key constituents of S. hemiphyllum extracts.
Cell viability and insulin secretion in culture with different glucose concentrations
Glucose toxicity to β-cells is caused by chronic exposure to extra-physiological glucose concentrations, which irreversibly damage pancreatic β-cells. However, β-cells can recover from glucose toxicity if the exposure time is limited to minutes or hours (Robertson et al. 2004). Interestingly, we observed no toxicity in RIN-5F cells incubated for 10 days in medium containing 5.6, 11 and 28 mM glucose. However, the insulin secretion from RIN-5F cells incubated with 11 and 28 mM glucose (39.2 ± 5.1 and 14.0 ± 0.9 ng/ml, respectively) were significantly lower than that with 5.6 mM glucose (64.2 ± 3.8 ng/ml; Fig. 1). Eizirik et al. (1992) incubated human pancreatic islet cells for 7 days with 5.6, 11 and 28 mM glucose, which showed no glucose-induced changes to islet DNA contents or morphology. However, islets cells incubated with 11 and 28 mM glucose showed a 45 % and 60 % decrease in insulin concentrations, respectively, compared with islets cells incubated with 5.6 mM glucose.
It is noteworthy that incubation of β-cells with extra-physiological glucose concentrations leads to progressive decreases in glucose-induced insulin secretion (Ling and Pipeleers 1996; Poitout et al. 1996; Roberston et al. 1992). Accordingly, we selected the 28 mM glucose condition to assess the anti-diabetic effects of S. hemiphyllum extracts.
Cell viability and insulin secretion in culture with S. hemiphyllum extracts
After incubation with 28 mM glucose for 10 days, RIN-5F cells were treated with 10, 100, or 250 μg/ml WES, EES, or AES for 24 h and showed no apparent cytotoxicity (Fig. 2). Zhang et al. (2008) also demonstrated that seaweed compounds were not cytotoxic to RIN-5F cells after 72 h.
To estimate anti-diabetic activities, we examined the stimulatory activities of WES, EES, and AES on insulin secretion from β cells. We used glybenclamide, a known anti-diabetic agent to stimulate pancreatic cells and cause insulin secretion, as a positive control. And 20 mg/ml glucose was used as the stimulant for supraphysiologic glucose β-cell (culture with 28 mM glucose medium for 10 days). The results are shown in Fig. 2. RIN-5F cells incubated with 28 mM glucose medium for 10 days lowered insulin secretory response to 20 mg/ml glucose. When the cells were treated with WES, EES and AES, it was found that 250 μg/ml WES, EES and AES elicited significant insulin secretion in comparison with the 20 mg/ml glucose group. In particular, 250 μg/ml AES stimulated insulin secretion to five times that of the control, showing similar efficacy to glibenclamide. Importantly, of all the S. hemiphyllum extracts in this study, AES had the highest polyphenol and fucoxanthin concentrations.
Previous studies suggested that polyphenols could stimulate insulin secretion via multiple mechanisms. Among these, epigallocatechin gallate and genistein have been shown to activate IRS2 and AMPK signaling (Cai and Lin 2009) and increase cAMP accumulation and insulin release, respectively, in β-cells (Ohno et al. 1993). Fucoxanthin supplementation of diabetic mice with markedly reduced plasma insulin levels ameliorated insulin resistance by suppressing pro-inflammatory cytokines (Hosokawa et al. 2010). Hence, in addition to inhibition of α-amylase and α-glucosidase, the strong antioxidant properties of polyphenols and fucoxanthin may underlie the anti-diabetic effects of S. hemiphyllum extracts (Fig. 3).
The effects of combined treatment with S. hemiphyllum extracts and glibenclamide on insulin secretion
Glibenclamide is available for the treatment of diabetes in several countries. However, some medications produce unwanted side effects when used in conjunction with glibenclamide. Therefore, effective anti-hyperglycemic agents that do not produce side effects are urgently required. In a previous study, extracts from Trigonella foenum graecum seeds (Lal et al. 2011) had significant hypoglycemic effects compared with glibenclamide, and combinations of these treatments produced greater improvements than either treatment alone. Thus, we examined the effects of the S. hemiphyllum extracts WES, EES, and AES in combination with glibenclamide. The dose-dependent effect of glibenclamide on insulin secretion is shown in Fig. 4. Co-treatment with 250 μg/ml EES or AES and 25 mg/ml glibenclamide significantly increased insulin secretion to a greater degree than the treatment with 50 mg/ml glibenclamide alone. The combination of 250 μg/ml AES and 25 mg/ml glibenclamide gave the maximum insulin secreting effect. Moreover, WES, EES, or AES did not exacerbate the side effects of glibenclamide, suggesting that AES could be used to increase pharmacological effects and decrease adverse effects of glibenclamide.
Effect of the combination of S. hemiphyllum extracts (WES, EES and AES) and glibenclamide on insulin secretion by RIN-m5F β cell cultivated with 28 mM/l glucose medium for 3 h (n = 5). Cells were treated with 10 μl of 250 μg/ml extracts, and with or without 10 μl of 25 mg/ml glybenclamide. *Significantly different from the 50 mg/ml glibenclamide group (p < 0.05)
Effects of combined treatment with amylin, S. hemiphyllum extracts, or glibenclamide on insulin secretion
The islet amyloid polypeptide amylin is localized in β-cell secretory granules and is co-secreted with insulin. It is known that amylin can inhibit glucose and non-glucose stimulated insulin secretion from pancreatic cells (Degano et al. 1993; Salas et al. 1995) and reduces insulin secretion following treatment with glibenclamide (Zhang et al. 2008). To examine the interactions of S. hemiphyllum extracts and amylin, we analyzed insulin secretion from RIN-5F cells and observed reduced insulin secretion after co-treatment with glibenclamide and amylin compared with that after treatment with glibenclamide alone. However, WES, EES, and AES had no influence on insulin secretion in the presence of amylin (Fig. 5). Although these data suggest an additive effect of WES, EES, and AES, the associated mechanisms remain unknown. We suggest that WES, EES, and AES influence amylin metabolism or insulin secretion in β-cells (Salas et al. 1995).
The effect of amylin on insulin secretion by RIN-m5F β cell in the presence of S. hemiphyllum extracts (WES, EES and AES) or glibenclamide cultivated with 28 mM/l glucose medium for 3 h (n = 5). Cells were treated with 10 μl of 250 μg/ml extracts or 50 mg/ml glibenclamide, and with or without 10 μl of 0.3 mg/ml amylin. *Significantly different between two parallel groups (p < 0.05)
This initial screening study shows that WES, EES, and AES have high polyphenol concentrations, with the highest fucoxanthin concentration found in AES. WES, EES, and AES inhibited α-amylase, sucrose, and maltase, stimulated insulin secretion, and had no side effect in conjunction with glibenclamide. Moreover, all extracts moderated the inhibition of insulin secretion caused by amylin. These data indicate that the S. hemiphyllum extracts WES, EES, and AES could be used to reduce glibenclamide doses, which may minimize adverse effects and enhance therapeutic efficacy.
References
Adefegha SA, Oboh G (2012) In vitro inhibition activity of polyphenol-rich extracts from Syzygium aromaticum (L.) Merr. & Perry (Clove) buds against carbohydrate hydrolyzing enzymes linked to type 2 diabetes and Fe2+-induced lipid peroxidation in rat pancreas. Asian Pac J Trop Biomed 2:774–781
Apostolidis E, Lee CM (2010) In vitro potential of phenolic antioxidant-mediated α-glucosidase and α-amylase inhibition. J Food Sci 75:H97–H102
Cai EP, Lin JK (2009) Epigallocatechin gallate (EGCG) and rutin suppress the glucotoxicity through activating IRS2 and AMPK signaling in rat pancreatic beta cells. J Agric Food Chem 57:9817–9827
Degano P, Silvestre RA, Salas M, Peiro E, Macrco J (1993) Amylin inhibits glucose-induced insulin secretion in a dose-dependent manner. Study in the perfused rat pancreas. Regul Pept 43:91–96
Eizirik D, Korbutt GS, Hellerstrom C (1992) Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the β-cell function. J Clin Invest 90:1263–1268
Hagerman AE (1988) Extraction of tannin from fresh and preserved leaves. J Chem Ecol 14:453–461
Hara Y, Honda M (1990) The inhibition of α-amylase by tea polyphenols. Agric Biol Chem 54:1939–1945
Heo SJ, Hwang JY, Choi JI, Han JS, Kim HJ, Jeon YJ (2009) Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potentα-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur J Pharmacol 615:252–256
Hosokawa M, Miyashita T, Nishikawa S, Emi S, Tsukui T, Beppu F, Okada T, Miyashita K (2010) Fucoxanthin regulates adipocytokine mRNA expression in white adipose tissue of diabetic/obese KK-A y mice. Arch Biochem Biophys 504:17–25
International Diabetes Federation (IDF) Diabetes Atlas (2009) 4th ed. International diabetes federation. Brussels, Belgium
ISO 14502-1 (2005) Determination of substances characteristic of green and black tea. Part 1: content of total polyphenols in tea. Colorimetric method using Folin-Ciocalteu reagent
Iwai K (2008) Antidiabetic and antioxidant effects of polyphenols in brown alga Ecklonia stolonifera in genetically diabetic KK-Ay mice. Plant Foods Hum Nutr 63:163–169
Jenkins DJ, Taylor RH, Goff DV, Fielden H, Misiewicz JJ, Sarson DL, Bloom SR, Alberti KG (1981) Scope and specificity of acarbose in slowing carbohydrate absorption in man. Diabetes 30:951–995
Kato A, Minoshima Y, Yamamoto J, Adachi I, Watson AA, Nash RJ (2008) Protective effects of dietary chamomile tea on diabetic complications. J Agric Food Chem 56:8206–8211
Kotake-Nara E, Kushiro M, Zhang H, Sugawara T, Miyashita K, Nagao A (2001) Carotenoids affect proliferation of human prostate cancer cells. J Nutr 131:3303–3306
Lal VK, Gupta PP, Tripathi P, Pandey A (2011) Interaction of aqueous extract of Trigonella foenum-graecum seeds with glibenclamide in streptozotocin induced diabetes rats. Am J Pharmacol Toxicol 6:102–106
Ling Z, Pipeleers DG (1996) Prolonged exposure of human β-cells to elevated glucose levels results in sustained cellular activation leading to a loss of glucose regulation. J Clin Invest 98:2805–2812
Maeda H, Tsukui T, Sashima T, Hosokawa M, Miyashita K (2008) Seaweed carotenoid, fucoxanthin, as a multi-functional nutrient. Asia Pac J Clin Nutr 17:196–199
Monami M, Luzzi C, Lamanna C, Chiasserini V, Addante F, Desideri CM, Masotti G, Marchionni N, Mannucci E (2006) Three-year mortality in diabetic patients treated with different combinations of insulin secretagogues and metformin. Diabetes Metab Res Rev 22:477–482
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Ohno T, Kato N, Ishii C, Shimizu M, Ito Y, Tomono S, Kawazu S (1993) Genistein augments cyclic adenosine 3′5′-monophosphate (cAMP) accumulation and insulin release in MIN6 cells. Endocr Res 19:273–285
Park HJ, Lee MK, Park YB, Shin YC, Choi MS (2011) Beneficial effects of Undaria pinnatifida ethanol extract on diet-induced-insulin resistance in C57BL/6 J mice. Food Chem Toxicol 49:727–733
Poitout V, Olson LK, Robertson RP (1996) Chronic exposure of βTC-6 cells to supraphysiologic concentrations of glucose decreases binding of the RIPE-3b1 insulin gene transcription activator. J Clin Invest 97:1041–1046
Porter LJ (1989) Condensed tannins. Natural Products of Woody Plants I. Springer-Verlag, Berlin, pp 651–690
Roberston RP, Zhang HJ, Pyzdrowski KL, Walseth TF (1992) Prevention of insulin mRNA levels and insulin secretion in HIT cells by avoidance of chronic exposure to high glucose concentrations. J Clin Invest 90:320–325
Robertson RP, Harmon J, Tran PO, Poitout V (2004) Beta-cell glucose, toxicity, lipotoxicity and chronic oxidative stress in type 2 diabetes. Diabetes 53:119–124
Salas M, Silvestre RA, Garcia-Hermida O, Fontela T, Rodriguez-Gallardo J, Macro J (1995) Inhibitory effect of amylin (islet amyloid polypeptide) on insulin response to non-glucose stimuli. Study in perfused rat pancreas. Diabete Metab 21:269–273
Serrano-Martín X, Payares G, Mendoza-León A (2006) Glibenclamide, a blocker of K+ATP channels, shows antileishmanial activity in experimental murine Cutaneous Leishmaniasis. Antimicrob Agents Chemother 50:4214–4216
Stauber JL, Jeffrey SW (1988) Photosynthetic pigments in fifty-one species of marine diatoms. J Phycol 24:158–172
Teixeira VL, Rocha FD, Houghton PJ, Kaplan MAC, Pereira RC (2007) α-Amylase inhibitors from Brazilian seaweeds and their hypoglycemic potential. Fitoterapia 78:35–36
Vittimberga FJ Jr, McDade TP, Perugini RA, Callery MP (1999) Sodium salicylate inhibits macrophage TNF-alpha production and alters MAPK activation. J Surg Res 84:143–149
Waterman PG, Mole S (1994) Analysis of phenolic plant metabolites. Blackwell Scientific Publications, Oxford
Weir GC, Laybutt DR, Kaneto H, Bonner-Weir S, Sharma A (2001) Beta-cell adaptation and decompensation during the progression of diabetes. Diabetes 50:S154–S159
Wong KH, Sam SW, Cheung PCK, Ang PO (1999) Changes in lipid profiles of rats fed with seaweed-based diets. Nutr Res 19:1519–1527
Yao O (2003) One Chinese medicine cures one illness. Linkingbooks, Taipei, pp 128–129
Zhang J, Tiller C, Shen J, Wang C, Girouard GS, Dennis D, Barrow CJ, Miao M, Ewart HS (2007) Antidiabetic properties of polysaccharide- and polyphenolic-enriched fractions from the brown seaweed Ascophyllum nodosum. Can J Physiol Pharmacol 85:1116–1123
Zhang D, Fujii I, Lin C, Ito K, Guan H, Zhao J, Shinohara M, Matsukura M (2008) The stimulatory activities of polysaccharide compounds derived from algae extracts on insulin secretion in vitro. Biol Pharm Bull 31:921–924
Zimmet P, Alberti K, Shaw J (2001) Global and societal implications of the diabetes epidemic. Nature 414:782–787
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hwang, PA., Hung, YL., Tsai, YK. et al. The brown seaweed Sargassum hemiphyllum exhibits α-amylase and α-glucosidase inhibitory activity and enhances insulin release in vitro. Cytotechnology 67, 653–660 (2015). https://doi.org/10.1007/s10616-014-9745-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-014-9745-9