Abstract
Many neural disorders are characterized by the loss of one or several types of neural cells. Human umbilical cord-derived mesenchymal cells (hUCMs) are capable of differentiating into neuron, astroglia-like and oligodendrocyte cell types. However, a reliable means of inducing the selective differentiation of hUCMs into neural cells in vitro has not yet been established. For induction of neural differentiation, hUCMs were seeded onto sterile glass slides and six various cocktails using a base medium (DMEM/LG) supplemented with 10 % FBS, retinoic acid (RA), dimethyl sulfoxide (DMSO), epidermal growth factor (EGF) and fibroblast growth factor (FGF) were used to compare their effect on neuronal, astrocyte and oligodandrocyte differentiation. The hUCMs were positive for mesenchymal markers, while they were negative for hematopoietic markers. Differentiation to adipogenic and osteogenic lineage was detected in these cells. Our data revealed that the cocktail consisting of DMEM/LG, FBS, RA, FGF, and EGF (DF/R/Fg/E group) induced hUCM cells to express the highest percentage of nestin, ß-tubulin III, neurofilament, and CNPase. The DF/Ds/Fg/E group led to the highest percentage of GFAP expression. While the expression levels of NF, GFAP, and CNPase were the lowest in the DF group. The least percentage of nestin and ß-tubulin III expression was observed in the DF/Ds group. We may conclude that FGF and EGF are important inducers for differentiation of hUCMs into neuron, astrocyte and oligodendrocyte. RA can induce hUCMs to differentiate into neuron and oligodendrocyte while for astrocyte differentiation DMSO had a pivotal role.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Umbilical cord is a disputed source of mesenchymal stromal cells (MSCs). MSCs isolated from Wharton’s Jelly (hUCMs (were shown to have surface markers, immune properties (Fong et al. 2011; Weiss et al. 2006), and differentiation potential towards cells with mesoderm offspring (Penolazzi et al. 2009; Schneider et al. 2010), including adipose, bone, cartilage, skeletal muscle, endothelium, cardiomyocyte and neuronal cells (Ishige et al. 2009; Leeb et al. 2009; Penolazzi et al. 2009; Wu et al. 2007), allowing replacement of ectodermal and mesodermal tissues (Schneider et al. 2010). Several reports have characterized the in vitro differentiation capabilities of hUCM cells (La Rocca et al. 2009) into neuron like cells (Abdulrazzak et al. 2010; Cao and Feng 2009; Hu et al. 2009; Zhang et al. 2009b). Early studies focused on the differentiation of MSCs into neurons and detection of astrocyte markers received only modest attention (Boucherie and Hermans 2009). But recent studies showed that hUCMs can be induced for selective differentiation into cells with the morphologic and immunophenotypic characteristics of oligodendrocyte precursor-like cells under culture conditions (Zhang et al. 2009a).
The first proof that mouse embryonic stem cells can be differentiated into multiple neural phenotypes in culture was reported by Bain and colleagues in 1995 on the base of using retinoic acid (Vawda 2008). Retinoic acid (RA), a derivative of vitamin A, one of the most important extrinsic neural inductive signals (Okada et al. 2004; Paschaki et al. 2012), has been used in combination with other elements to induce MSC differentiation into neural cells (Scintu et al. 2006). Various growth factors (Jori et al. 2005), such as, basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) are involved in transformation and morphological differentiation of stem cells into neurons (Fu et al. 2004; Rasmussen et al. 2011). Embryonic stem cells can be induced to differentiate into neurons and glia cells by applying a modified medium enriched with RA or bFGF (Kim et al. 2011; Mitchell et al. 2003). bFGF induces the cells to express a neural phenotype, oligodendrocytes and astrocytes (Malkowski et al. 2007; Sobolewski et al. 2005). Also, dimethyl sulfoxide (DMSO) can initiate a coordinated differentiation program in various cell types (Adler et al. 2006). Supplementation of DMEM-LG with β-mercaptoethanol, DMSO, KCL and RA induced bone marrow cells to express a neural phenotype (Mammadov et al. 2011). Also treatment with neurobasal medium, bFGF, EGF plus B27 and RA induced Wharton`s jelly cells to differentiate into neuron-like cells. Markers for oligodendrocytes and astrocytes were also detected in Wharton`s jelly cells (Zhang et al. 2009a).
In a review article on neural cell differentiation methods Schwartz et al. (2008) showed many variations in the use of the materials to induce the differentiation of mesenchymal cells into neural cells. FGF and EGF have important role in the maintenance and expansion of differentiated cells (Schwartz et al. 2008). What is important is none of the methods was used to induce the hUCMs differentiation into neural cells. So, we decided in this study to compare the protocols suggested for neural induction of hUCMs in vitro, and to provide protocols to achieve considerable number of neuron, astrocyte and oligodendrocyte like cells from hUCM cells.
Materials and methods
Isolation of human umbilical cord matrix-derived mesenchymal cells
All materials were purchased from Sigma Company (Sigma-Aldrich, St. Louis, MO, USA) unless stated otherwise. Ethical approval was obtained from the Institutional ethical review board (approval number 69-1780) at Kerman University of Medical Sciences, Kerman, Iran. Umbilical cords were obtained from the patients delivering full-term infants by Caesarian section after a written consent had been obtained (n = 5). The cord was cut into 3–5 cm long pieces using sterile sharp blade. Blood vessels were removed from each piece after incising the cord lengthwise and Wharton’s jelly was carefully separated from the amniotic membrane. WJ fragments were placed in 1 mg/mL collagenase type B for 3 h at 37 °C. Then the fragments were washed with PBS and 0.25 mg/mL trypsin was added for 15 min at 37 °C with agitation. Then the larger pieces were removed and the isolated cells were washed with PBS, followed by seeding of the cells onto the surface of a culture dish with DMEM/LG supplemented with 10 % (V/V) FBS penicillin (100 U/mL), streptomycin (100 μg/mL), and amphotericin-B (2.5 μg/mL) for 1 week. Within this period, the cells were fed by the replacement with fresh medium every other day until the cells reached an approximately 80 % confluence.
Preparation of mesenchymal stromal cells
The isolation of cells with stem cell-like morphology (small, round cells and spindle-shaped cells) was enhanced by selective elimination of the population of tightly adhered cells because it seemed that differentiated cells separate with retardation at the first passage. Then this aware cell selection was followed by incubating the remaining cells in trypsin/EDTA for 3 min so only the cells that detached in this period of time were resuspended in growth medium and seeded to the next flask. This method was repeated for subsequent passages (Carlin et al. 2006).
Flow cytometry
After the fourth passage, the cells were prepared at a concentration of 1 × 105 cells/mL in DMEM/LG with 10 % FBS. The cells were washed with PBS, fixed and incubated for 15 min at 4 °C with a 1:9 dilution of normal goat serum in PBS to block nonspecific binding of the antibody. The cells were labeled with the following antibodies: FITC-conjugated anti-CD44, FITC-conjugated anti-CD34 (Chemicon; Temecula, CA, USA), FITC-conjugated anti-CD45 (Ediscience; USA), PE-conjugated-anti-CD73 (BD; San Jose, CA, USA), PE-conjugated anti-CD90 (Dako, Glostrup, Denmark) and PE-conjugated anti-CD105 (R&D; Minneapolis, MN, USA) for 1 h. The cells were washed with 2 % FBS in PBS and analyzed using FACS Calibur (BD = Becton Dickenson, USA) machine. The control population was stained with matched isotype antibodies (FITC-conjugated and PE-conjugated mouse IgG monoclonal isotype standards), which were confirmed by positive fluorescence of limbal samples. At least 10,000 events were recorded for each sample and data were analyzed using WinMDI software (USA).
Cell differentiation procedures
The umbilical cord MSCs at fourth passage were induced to differentiate into osteocyte and adipocyte. The cells were cultured in DMEM/LG medium which consisted of either osteogenic (10 nM dexamethasone, 50 μg/mL ascorbate-phosphate, and 10 mM β-glycerophosphate), or adipogenic (100 nM dexamethasone, 50 μg/mL ascorbate-phosphate, and 50 μg/mL indomethacin) additives. Adipogenic differentiation was detected by Oil Red O staining while osteogenic differentiation was detected by Alizarin Red-S staining.
Induction of neural differentiation
At the fourth passage, the hUCM cells were seeded at a density of 1 × 104 cells/mL onto clean sterilized glass slides. After 1 h incubation at 37 °C, the cells were treated with different protocols for 2 weeks. The media were refreshed every other day. DMEM/LG and FBS was the basic medium in all groups. Dimethyl sulfoxide (DMSO), retinol acetate (RA), fibroblast growth factor (FGF), and epidermal growth factor (EGF) were used to induce hUCM cells into neural lineages as is shown in Table 1.
Immunocytochemistry
Two weeks after the onset of treatments, the differentiated cells were characterized by immunocytochemistry. The slides were fixed in 4 % paraformaldehyde for 15 min followed by three times washing in PBS. For intracellular antigens the cells were permeabilized with PBS containing 0.2 % Triton X-100 for 10 min and were washed two times in Tris buffered saline (TBS). Then, the samples were incubated for 45 min with anti-nestin (1/200, Chemicon MAB5326), anti-neurofilament (Chemicon MAB1615), anti-β-tubulin III (1/100, Chemicon 16230), anti-CNPase (1/200, Chemicon MAB326) and anti-GFAP (1/400, Chemicon MAB360) antibodies. After two washes in PBS the antigen was localized with a peroxidase kit and DAB chromogenic substrate solution (DAKO). The slides were counterstained with hematoxylin (DAKO). Negative controls were considered by omitting the primary antibody. Two hundred cells in random fields were examined in each culture slide at ×10 magnification. All slides were studied using an optical microscope (Nikon ys100, Nikon, Tokyo, Japan). The fraction of positive cells was calculated by counting 10 non-overlapping microscopic fields for each slide in at least three separate experiments.
Statistical analysis
Data are presented as percentage of positive cells in all groups. The level of significance was assessed by Chi Square test. Statistical significance was assigned at a P value ≤0.05.
Results
Cell morphology
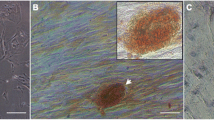
hUCM cells appeared as spherical, star like or elongated flat fibroblast like cells with some granules on their surfaces and cell bodies without any protrusion and networks among the cells. By our selective protocol for cell passage, after the second passage the morphology of the cells appeared to have become homogenous. Our method increased the proportion of cells with stem cell-like morphology as well as their ability to form colonies. The cells that remained adherent to the flask at each passage, had large and flat morphology and they produced few colonies as compared to the passaged cells (Fig. 1).
Flow cytometry
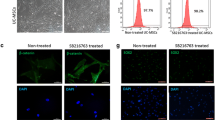
Human umbilical cord cells were negative for hematopoietic cells surface markers, CD34 and CD45. While, the mesenchymal stromal cell markers; CD44, CD73, CD90 and CD105, were positive in these cells (Fig. 2).
Flow cytometry results. The red histograms are the isotype control-stained cells, the black histograms are the antibody-stained cells. The percentage of cells staining for each marker is provided. hUCM cells were negative for heamatopoietic markers; CD34 and CD45. These cells were positive for mesenchymal stem cell markers; CD44, CD73, CD90 and CD105. (Color figure online)
Osteogenic and adipogenic differentiation
By the end of the third week, the cells which were induced with adipogenic medium contained numerous oil-red positive lipid droplets (Fig. 3a). Similarly, the MSCs became alizarin-red positive (Fig. 3c). Non-treated control cultures did not show spontaneous adipocyte or osteoblast transformation even after 3 weeks of cultivation (Fig. 3b, d).
Immunocytochemistry
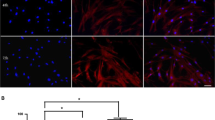
After 2 weeks of hUCM cells culture with various cocktails, immunocytochemistry test revealed that induced cells express different differentiation markers of neural lineage unequally (Fig. 4). The cocktail consisting of DMEM/LG, FBS, RA, FGF, and EGF (DF/R/Fg/E group) expressed the highest percentage of nestin (69.6 %), ß-tubulin III (76 %), neurofilament (89 %), and CNPase (16.3 %) positive cells. While, in DF/Ds/Fg/E group the highest percentage of GFAP expression (36 %), as an astrocyte marker, was detected. Also, in this group and the DF/Ds/Fg/E group, the highest cell growth was observed (data not shown). The expression level of NF, GFAP, and CNPase was at least in the DF group. Also the least percentage of nestin and ß-tubulin III expression was observed in the DF/Ds group (Table 2). Chi Square test showed that the expression level of nestin and CNPase in all groups was significantly higher than in the control group (DF group). Significantly higher expression of ß-tubulin III was detected in all groups compared with the control, expect in the DF/R group. While, NF and GFAP were expressed significantly higher in all groups compared with the control, expect in the DF/R/Ds group and the DF/Ds group, respectively (Fig. 5). In addition, the evaluation of staining intensity in the ß-tubulin III positive cells showed a stable intensity among the groups with a detectable staining in part of their cytoplasm (+), the whole cytoplasm (++) and the whole cytoplasm and the cell membrane (+++) while most of the cells exhibited a detectable staining in part of their cytoplasm in CNPase antibody among all groups. The rest of the antibodies showed very variant intensities among the groups (Fig. 6).
Differentiated hUCMs expressed specific neural markers determined by immunocytochemistry. Nuclei were stained in Nestin (a) and β-tubulin III (c) positive cells. NF (e) was positive specially in cells being characterized by bipolar location, whereas GFAP (g) and CNPase (i) positive cells are stained across the whole of the cells (arrows). Mouse brain was stained as positive control for nestin (b), β-tubulin III (d), GFAP (h) and CNPase (j) and undifferentiated hUCMs were used as negative control (f). Original magnification a, c, e, g and i ×400; b, d, f, h and j ×100
Semi-quantification of immunostaining data for different neural and glial markers expression in various groups. (+) = part of cytoplasm stained, (++) = the whole cytoplasm stained (+++) = the whole cytoplasm and cell membrane stained. (*, ** and *** show significant differences of intensity in an antibody expression). Significant differences of intensity are seen in all antibodies in DF/D/Fg/E group
Discussion
Understanding mesenchymal stromal cells propensity to differentiate into various lineages is a fundamental factor for the development of successful cell-based therapies (Nekanti et al. 2010). In this matter, the possible acquisition of glial functions by grafted stem cells is now considered as a relevant explanation for the benefit of this method in CNS disorders (Boucherie and Hermans 2009). In addition, the investigation on in vitro neural differentiation of stem-like cells can lead to the understanding of some mechanisms behind the neural tissue development (Hadinger et al. 2009). So, in this study, the ability of several cocktails to differentiate hUCM cells into neural cells was compared. We found the cocktail consisting of DMEM/LG, FBS, RA, FGF, and EGF (DF/R/Fg/E) induced hUCM cells to express the highest percentage of neural and oligodendrocyte markers and the cocktail consisting of DMEM/LG, FBS, DMSO, FGF, and EGF (DF/Ds/Fg/E) induced the highest percentage of astrocyte marker.
In this study, we selected the UC-derived MSCs as a new source of stem cells (Wu et al. 2007), Studies have shown that MSCs can easily be cultivated for more than 3 months without any senescence evidence and reduction of differentiation potential (Leeb et al. 2009). Of course, some factors such as; basal nutrients, cell density, growth factors and cytokines, all play significant roles in MSC differentiation (Bobis et al. 2006). In the present investigation the effect of the defined inductive cocktails on neural and glial differentiation was inspected and also the roles of individual as well as combination of components from cocktails were examined for neural markers expression. Through the experiments we used DMEM/LG as base medium because our unpublished data and also Sotiropoulou et al. (2006) have shown that low glucose concentration in DMEM-based media consistently supported MSC growth (Sotiropoulou et al. 2006), and furthermore DMEM/LG was identified as an ideal performer for cell differentiation (Lund et al. 2009).
In our study, the DF cocktail without any inducer resulted in a considerable rate of expression of nestin, β-tubulin, NF, GFAP and even CNpase (Table 2). These data are in agreement with other studies that untreated umbilical mesenchymal stromal cells express a number of neural markers including nestin and β-tubulin III (Vawda 2008) and glial proteins spontaneously (Fu et al. 2004). Recently, some researchers had claimed that neural differentiation of MSCs might be a reflection of cellular stress in comparison with cells entering a true differentiation program. But, Tio et al. (2010) have suggested that the expression of neural markers in MSCs is a consequence of differentiation rather than stress. A sub-population of UCM cells exhibits neuronal morphology and expresses certain neuronal phenotype markers in culture, in the absence of specialized inducing factors (Deng et al. 2006; Medicetty 2005; Weiss et al. 2003). Maybe they are primed to differentiate along a neural program.
Wislet-Gendebien et al. (2005) believed that nestin expressed by MSCs is a necessary factor for the emergence of neuronal differentiation of the MSCs (Wislet-Gendebien et al. 2005). Peng et al. (2010) found that a few number of cells was nestin positive in adherent hUCMs (2.25 ± 0.42 %) (Peng et al. 2010). While, the results from this study showed that nestin expression in untreated cells (DF group) was much higher (46 %). It seems that the long incubation time in cell culture, in our work, has an important role in expression of nestin. β-tubulin III is a classic marker of immature and mature neurons that was expressed in 50–70 % of various cell types in untreated medium. Even further treatment with neural induction medium did not increase (73–85 %) β-tubulin III positive hMSCs significantly (Vawda 2008). In this study, β-tubulin III expression in untreated cells (DF group) was nearly identical to the induced cells. FBS contains significant levels of RA (Okada et al. 2004). We believe that the RA level in FBS is sufficed for nestin and β-tubulin III expression and even extra level of RA decreased nestin expression but, did not affect β-tubulin III expression. Of course, long-term presence of RA (14 days) did not influence the rate of neural positive markers (Hadinger et al. 2009). But, Fu et al. (2004) believed that prolonged treatment with RA (more than 12 days) forms cell to cell contacts and constructs the appearance of a network.
Basic fibroblast growth factor and retinoic acid differentially influence human motor neuron differentiation by mechanisms which still have remained undefined (Shin et al. 2005). The results from this study showed that a 14 days induction of hUCM cells with RA in combination with FGF and EGF had the greatest influence on nestin and β-tubulin III expression. Moreover, the presence of DMSO alone decreased the expression of nestin and β-tubulin III, but DMSO in combination with FGF and EGF induced hUCMs to express considerable level of nestin and β-tubulin. Maximum non-effective solvent concentration of DMSO on induction of MSC differentiation has been determined as high as 0.25 % as previously reported (Adler et al. 2006). So we used a concentration of 0.5 % DMSO in our cocktails for induction of neural differentiation.
The morphology of NF-positive differentiated mesenchymal cells can be bipolar, multipolar or mesenchymal (Fu et al. 2004). In the present study although hUCM cells did not show much neurite-like morphology, they were able to upregulate neural markers expression. NF expression, in this study, was different from nestin and β-tubulin III expression among the groups. RA had an evident effect on NF expression. Combination of RA with FGF and EGF resulted in higher expression of NF. Contrary to us, Portmann-Lanz et al. (2010) reported that RA had little effects on NF expression. While, the combination of IBMX with RA induced NF-L expression. It is suggested that there were some synergistic interactions between IBMX and RA for NF-L expression (Tio et al. 2010).
Great controversies exist between different studies when mesenchymal cell induction for astrocyte differentiation is considered. Karahuseyinoglu et al. (2007) reported that GFAP expression was negative in both induced and noninduced hUCM cells (Karahuseyinoglu et al. 2007). Also, Portmann-Lanz et al. (2010) have shown that none of the method of neural induction resulted in the generation of GFAP-positive astrocytes. In contrast, GFAP-positive cells were observed in less than 5 % of untreated Wharton’s jelly cells (Fu et al. 2004). While, the expression of these cells was slightly higher after induction with bFGF, DMSO and BHA (Mitchell et al. 2003). Using a three-step neural induction protocol of bFGF, β-mercaptoethanol, neurotrophic factor-3 (NT-3), nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) for 14 days, 32 % of hUCMs differentiated into GFAP-positive cells (Fan et al. 2010). The results from this study showed that the proportion of GFAP-positive cells in untreated cells was less than induced cells in DF/Ds/Fg/E group. GFAP can also be expressed by immature neural progenitor cells (Schwindt et al. 2009). Long-term (14 days) exposure to RA dramatically reduced the GFAP-positive cells. It has been shown that RA can prevent the formation of GFAP-positive astrocytes if added to the defined phases of in vitro neural differentiation (Hadinger et al. 2009). In this study, RA alone did not increase the GFAP expression, but combination of RA with DMSO increased the rate of GFAP-positive cells. We found that DMSO alone or in combination with RA or FGF and EGF had a positive effect on GFAP expression in hUCM cells.
This study showed that the most appropriate cocktail for differentiation of hUCMs to oligodendroglia was the DF/R/Fg/E cocktail. Expression of CNPase has been reported to be nearly identical in untreated, bFGF-treated, and fully induced Wharton’s jelly cells (Mitchell et al. 2003). In this study, untreated cells (DF group) contained the least (4 %) CNPase positive cells compared with the other treatments. Induction of hUCM cells with RA resulted in 8.6 % CNPase expression. When DMSO was added to RA the proportion of CNPase positive cells increased to 13.6 %. Moreover, addition of FGF and EGF to RA resulted in the highest CNPase expression (16.3 %). The cellular response to growth factors is not predefined or fixed, but to some extent it depends on some intrinsic and extrinsic influences (Vawda. 2008). Neural differentiation of placental stem cells with RA plus hBDNF resulted in 5–20 % of oligodendrocytic cells (Portmann-Lanz et al. 2010). It has been reported that bFGF increased the proliferative potential of MSCs (Schwindt et al. 2009; Sotiropoulou et al. 2006). We suggest that the increased cell density with FGF and EGF can lead to cells with a higher positive CNPase level. Portmann-Lanz et al. (2010) also believed that higher plating densities resulted in a greater proportion of oligodendrocytes. Moreover, in this study like in a previous study the induced cells had a smaller cell body with several minor extensions. Portmann-Lanz et al. (2010) believed that these cells were immature oligodendrocytes. In the developing CNS, the manifestation of the microglial phenotypes is delayed behind the formation of neurons (Hadinger et al. 2009). Whether prolongation of the treatment period may result in more glial cells requires further investigations.
We may conclude that untreated human umbilical cord mesenchymal cells appear to have an innate neural differentiation potential. We also conclude differentiation of human umbilical cord-derived mesenchymal cells into various neural phenotypes is highly dependent on inducing agents.
Abbreviations
- hUCM:
-
Human umbilical cord-derived mesenchymal cells
- RA:
-
Retinoic acid
- DMSO:
-
Dimethyl sulfoxide
- EGF:
-
Epidermal growth factor
- FGF:
-
Fibroblast growth factor
- MSCs:
-
Mesenchymal stromal cells
- bFGF:
-
Basic fibroblast growth factor
References
Abdulrazzak H, Moschidou D, Jones G, Guillot VP (2010) Biological characteristics of stem cells from foetal, cord blood and extraembryonic tissues. J R Soc Interface 7, Suppl 6:S689–S706
Adler S, Pellizzer C, Paparella M, Hartung T, Bremer S (2006) The effects of solvents on embryonic stem cell differentiation. Toxicol In Vitro 20:265–271
Bobis S, Jarocha D, Majka M (2006) Mesenchymal stem cells: characteristics and clinical applications. Folia Histochem Cytobiol 44:215–230
Boucherie C, Hermans E (2009) Adult stem cell therapies for neurological disorders: benefits beyond neuronal replacement? J Neurosci Res 87:1509–1521
Cao FJ, Feng SQ (2009) Human umbilical cord mesenchymal stem cells and the treatment of spinal cord injury. Chin Med J (Engl) 122:225–231
Carlin R, Davis D, Weiss M, Schultz B, Troyer D (2006) Expression of early transcription factors Oct-4, Sox-2 and Nanog by porcine umbilical cord (PUC) matrix cells. Reprod Biol Endocrinol 4:8
Deng J, Petersen BE, Steindler DA, Jorgensen ML, Laywell ED (2006) Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells 24:1054–1064
Fan CG, Zhang QJ, Zhou JR (2010) Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev 7:195–207
Fong CY, Chak LL, Biswas A, Tan JH, Gauthaman K, Chan WK, Bongso A (2011) Human Wharton’s jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev 7:1–16
Fu YS, Shih YT, Cheng YC, Min MY (2004) Transformation of human umbilical mesenchymal cells into neurons in vitro. J Biomed Sci 11:652–660
Hadinger N, Varga BV, Berzsenyi S, Kornyei Z, Madarasz E, Herberth B (2009) Astroglia genesis in vitro: distinct effects of retinoic acid in different phases of neural stem cell differentiation. Int J Dev Neurosci 27:365–375
Hu S, Zhang J, Hu X, Hu R, Luo H, Li F, Xia Y, Li J, Lin J, Zhu G, Feng H (2009) In vitro labeling of human umbilical cord mesenchymal stem cells with superparamagnetic iron oxide nanoparticles. J Cell Biochem 108:529–535
Ishige I, Nagamura-Inoue T, Honda MJ, Harnprasopwat R, Kido M, Sugimoto M, Nakauchi H, Tojo A (2009) Comparison of mesenchymal stem cells derived from arterial, venous, and Wharton’s jelly explants of human umbilical cord. Int J Hematol 90:261–269
Jori FP, Napolitano MA, Melone MA, Cipollaro M, Cascino A, Altucci L, Peluso G, Giordano A, Galderisi U (2005) Molecular pathways involved in neural in vitro differentiation of marrow stromal stem cells. J Cell Biochem 94:645–655
Karahuseyinoglu S, Cinar O, Kilic E, Kara F, Akay G, Demiralp D, Tukun A, Uckan D, Can A (2007) Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells 25:319–331
Kim ES, Kim GH, Kang ML, Kang YM, Kang KN, Hwang KC, Min BH, Kim JH, Kim MS (2011) Potential induction of rat muscle-derived stem cells to neural-like cells by retinoic acid. J Tissue Eng Regen Med 5:410–414
La Rocca G, Anzalone R, Corrao S, Magno F, Loria T, Lo Iacono M, Di Stefano A, Giannuzzi P, Marasa L, Cappello F, Zummo G, Farina F (2009) Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem Cell Biol 131:267–282
Leeb C, Jurga M, McGuckin C, Moriggl R, Kenner L (2009) Promising new sources for pluripotent stem cells. Stem Cell Rev 6:15–26
Lund P, Pilgaard L, Duroux M, Fink T, Zachar V (2009) Effect of growth media and serum replacements on the proliferation and differentiation of adipose-derived stem cells. Cytotherapy 11:189–197
Malkowski A, Sobolewski K, Jaworski S, Bankowski E (2007) FGF binding by extracellular matrix components of Wharton’s jelly. Acta Biochim Pol 54:357–363
Mammadov B, Karakas N, Isik S (2011) Comparison of long-term retinoic acid-based neural induction methods of bone marrow human mesenchymal stem cells. In Vitro Cell Dev Biol Anim 47:484–491
Medicetty S (2005) Effect of umbilical cord matrix stem cells on Parkinson’s disease model rats. PhD thesis. Kansas State, Manhattan
Mitchell K, Weiss M, Mitchell B, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T, Troyer D (2003) Matrix cells from Wharton’s Jelly form neurons and glia. Stem Cells 21:50–60
Nekanti U, Rao VB, Bahirvani AG, Jan M, Totey S, Ta M (2010) Long-term expansion and pluripotent marker array analysis of Wharton’s jelly-derived mesenchymal stem cells. Stem Cells Dev 19:117–130
Okada Y, Shimazaki T, Sobue G, Okano H (2004) Retinoic-acid-concentration-dependent acquisition of neural cell identity during in vitro differentiation of mouse embryonic stem cells. Dev Biol 275:124–142
Paschaki M, Lin SC, Wong RL, Finnell RH, Dolle P, Niederreither K (2012) Retinoic acid-dependent signaling pathways and lineage events in the developing mouse spinal cord. PLoS One 7:e32447
Peng J, Wang Y, Zhang L, Zhao B, Zhao Z, Chen J, Guo Q, Liu S, Sui X, Xu W, Lu S (2010) Human umbilical cord Wharton’s jelly-derived mesenchymal stem cells differentiate into a Schwann-cell phenotype and promote neurite outgrowth in vitro. Brain Res Bull 84:235–243
Penolazzi L, Vecchiatini R, Bignardi S, Lambertini E, Torreggiani E, Canella A, Franceschetti T, Calura G, Vesce F, Piva R (2009) Influence of obstetric factors on osteogenic potential of umbilical cord-derived mesenchymal stem cells. Reprod Biol Endocrinol 7:106
Portmann-Lanz CB, Schoeberlein A, Portmann R, Mohr S, Rollini P, Sager R, Surbek DV (2010) Turning placenta into brain: placental mesenchymal stem cells differentiate into neurons and oligodendrocytes. Am J Obstet Gynecol 202:294 e211–294 e291
Rasmussen MA, Hall VJ, Carter TF, Hyttel P (2011) Directed differentiation of porcine epiblast-derived neural progenitor cells into neurons and glia. Stem Cell Res 7:124–136
Schneider RK, Pullen A, Kramann R, Bornemann J, Knuchel R, Neuss S, Perez-Bouza A (2010) Long-term survival and characterisation of human umbilical cord-derived mesenchymal stem cells on dermal equivalents. Differentiation 79:182–193
Schwartz PH, Brick DJ, Stover AE, Loring JF, Muller FJ (2008) Differentiation of neural lineage cells from human pluripotent stem cells. Methods 45:142–158
Schwindt TT, Motta FL, Gabriela FB, Cristina GM, Guimaraes AO, Calcagnotto ME, Pesquero JB, Mello LE (2009) Effects of FGF-2 and EGF removal on the differentiation of mouse neural precursor cells. An Acad Bras Cienc 81:443–452
Scintu F, Reali C, Pillai R, Badiali M, Sanna MA, Argiolu F, Ristaldi MS, Sogos V (2006) Differentiation of human bone marrow stem cells into cells with a neural phenotype: diverse effects of two specific treatments. BMC Neurosci 7:14
Shin S, Dalton S, Stice SL (2005) Human motor neuron differentiation from human embryonic stem cells. Stem Cells Dev 14:266–269
Sobolewski K, Malkowski A, Bankowski E, Jaworski S (2005) Wharton’s jelly as a reservoir of peptide growth factors. Placenta 26:747–752
Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M (2006) Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells 24:462–471
Tio M, Tan KH, Lee W, Wang TT, Udolph G (2010) Roles of db-cAMP, IBMX and RA in aspects of neural differentiation of cord blood derived mesenchymal-like stem cells. PLoS One 5:e9398
Vawda R (2008) Characterisation and neurogenic potential of stem cells from the human umbilical cord matrix. PhD thesis. Imperial College London, London
Weiss ML, Mitchell KE, Hix JE, Medicetty S, El-Zarkouny SZ, Grieger D, Troyer DL (2003) Transplantation of porcine umbilical cord matrix cells into the rat brain. Exp Neurol 182:288–299
Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D (2006) Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells 24:781–792
Wislet-Gendebien S, Hans G, Leprince P, Rigo JM, Moonen G, Rogister B (2005) Plasticity of cultured mesenchymal stem cells: switch from nestin-positive to excitable neuron-like phenotype. Stem Cells 23:392–402
Wu KH, Yang SG, Zhou B, Du WT, Gu DS, Liu PX, Liao WB, Han ZC, Liu YL (2007) Human umbilical cord derived stem cells for the injured heart. Med Hypotheses 68:94–97
Zhang HT, Fan J, Cai YQ, Zhao SJ, Xue S, Lin JH, Jiang XD, Xu RX (2009a) Human Wharton’s jelly cells can be induced to differentiate into growth factor-secreting oligodendrocyte progenitor-like cells. Differentiation 79:15–20
Zhang L, Zhang HT, Hong SQ, Ma X, Jiang XD, Xu RX (2009b) Cografted Wharton’s jelly cells-derived neurospheres and BDNF promote functional recovery after rat spinal cord transection. Neurochem Res 34:2030–2039
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salehinejad, P., Alitheen, N.B., Ali, A.M. et al. Neural differentiation of human umbilical cord matrix-derived mesenchymal cells under special culture conditions. Cytotechnology 67, 449–460 (2015). https://doi.org/10.1007/s10616-014-9703-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-014-9703-6