Abstract
This study aimed to provide a preliminary test of the efficacy of a brief cognitive bias modification program for reducing approach bias in adult smokers motivated to quit. Participants were 52 smokers who were randomly assigned to four sessions of approach bias modification training (AAT) or sham training. Participants were asked to make a self-guided quit attempt upon completion of the final training session. Approach bias was assessed at baseline and at the end of each session, and days abstinent was assessed 1-week following the quit attempt. Individuals assigned to the AAT training condition evidenced significantly greater reductions in approach bias relative to those in the sham condition (p < .001). Baseline approach bias did not moderate the between-group effect (ps > 0.41); however, higher levels of approach bias at baseline were associated with greater approach bias reduction over time irrespective of condition (p < .001). Consistent with hypothesis, the reduction in approach bias during the intervention period was significantly related to the number of days abstinent following the quit attempt (p = .033). The present study extends recent work in alcohol use disorders by showing that approach bias reduction, in this case for smoking-related stimuli, may also facilitate smoking cessation. Clinical and research implications are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tobacco use remains the most preventable cause of disease, disability, and death in the United States, accounting for nearly 1 in 5 deaths each year (US Department of Health and Human Services 2014). While most smokers desire to quit, 75–80% of those who attempt to quit relapse (Zhou et al. 2009). The most recent guidelines for clinical practice on treating tobacco use and dependence state that, while progress has been made in terms of treatment development and dissemination, there is still a need for innovative, potent strategies for smoking cessation (Fiore et al. 2008).
Dual process models propose that addiction arises from an imbalance between two distinct, yet interacting, systems: the impulsive and reflective systems (Stacy and Wiers 2010; Wiers et al. 2010). The impulsive system relies on associative memory and often operates unconsciously and is difficult to control. Conversely, the reflective system is limited in capacity and relies on symbolic processing and often incorporates flexible learning (Wiers et al. 2013a). Friese, Hofmann, and Wiers (2011) have used a “horse and rider” metaphor to describe the interaction between these two systems, such that the horse (i.e., the impulsive tendencies) can be controlled by the rider (i.e., the reflective processes) should the rider acquire the necessary skills and strength. This metaphor underscores the rationale for using interventions like cognitive-behavioral treatment (CBT), which target reflective systems, but also the potential importance of interventions that target the automated, impulsive, implicit processes (Machulska et al. 2016).
The present study represents a next-step in research testing the efficacy of approach bias modification for smoking cessation. The research was guided by the following findings. First, approach bias, defined as the automatically activated action tendency to approach substance-related stimuli (Wiers et al. 2013a), is an implicit process associated with the maintenance of addiction. The bias has been found in problem users of alcohol and cannabis (Cousijn et al. 2011; Field et al. 2008) as well as smokers (Machulska et al. 2015; Wiers et al. 2013b). Second, accumulating evidence suggests that approach bias modification may facilitate therapeutic outcomes for alcoholic patients. Specifically, Wiers and colleagues showed that training alcoholic patients to push vs. pull a joystick when presented with pictures depicting alcoholic stimuli on a computer screen leads to a reduction in approach bias and a significant reduction in relapse at 1-year follow-up (Wiers et al. 2011; Eberl et al. 2013). Third, in an initial study with inpatient smokers, Machulska and colleagues (2016) showed evidence for an effect of approach bias modification on cigarette consumption, although no evidence for a reduction in approach bias as the mechanism of action. Together, these initial findings suggest that an implicit process like approach bias may be an important treatment target. To develop this application for smoking cessation specifically, it is important to test whether approach bias modification leads to a reduction in approach bias in treatment-seeking smokers and whether such a modification impacts smoking abstinence.
This pilot study aimed to provide an initial test of the efficacy of approach bias modification for engaging the putative treatment target and facilitating smoking cessation. We randomly assigned 52 treatment-seeking smokers to either four sessions of approach bias modification training (AAT training) or four sessions of placebo (sham training) prior to making a self-guided quit attempt. By restricting the intervention procedures to approach bias modification, this initial study among motivated treatment-seeking smokers can isolate the effects of the bias modification program. We assessed approach bias at baseline and at each of the four training sessions and measured days abstinent during a one-week follow-up after the quit attempt, as per recent recommendations for initial efficacy testing for novel smoking cessation interventions (Perkins 2014). We tested the following hypotheses: (1) persons assigned to the training condition would evidence greater reductions in approach bias relative to those assigned to the placebo condition; (2) initial approach bias would moderate the between-group effect on approach bias reduction, such that the effects would be greater among those evidencing a larger initial bias at baseline, as has been observed in other cognitive bias modification research (Amir, Taylor, & Donahue, 2011); and (3) greater reductions in approach bias would be associated with more days abstinent during the week following the quit attempt.
Method
Participants
Eligible participants were 52 adult smokers (Mage = 36.0, SD = 11.8). Participants were recruited from the Austin, Texas community through the use of fliers throughout the community and internet advertising (e.g., Craigslist). The study was advertised and presented as a potential novel smoking cessation intervention. In order to isolate treatment effects, participants were not enrolled if they were currently participating in any form of smoking cessation intervention. Eligibility criteria included: (1) adult daily smoker for at least 6 months (minimum of 8 cigarettes per day); (2) motivated to quit smoking (endorsing at least 5 on a 10-point scale); (3) interest in making a serious quit attempt within the next month without professional assistance or nicotine replacement therapy; and (4) not having decreased the number of consumed cigarettes by more than half in the last 6 months.
Participants were predominantly White (78.9%), 11.5% were Black/African American, 5.8% were Asian, 1.9% were Native Hawaiian or Pacific Islander, and 1.9% endorsed “other.” Overall, participants had a diverse educational background: 19.2% had completed graduate school, 36.5% had completed college, 34.6% had some college, and 9.6% had completed high school. The sample was highly motivated to quit smoking (M = 8.1 [on 0–10 Likert scale], SD = 1.6). Participants smoked an average of 13.7 (SD = 7.1) cigarettes per day. The sample endorsed moderate nicotine dependence as indicated by average scores on the Fagerström test for nicotine dependence of 4.9 (SD = 2.4; FTND; Heatherton et al. 1991).
Measures
Motivation to Quit Smoking Participants rated their motivation to quit smoking on a scale of 1–10 at screen.
Nicotine Dependence The Fagerström test for nicotine dependence (FTND; Heatherton et al. 1991) was administered at screen to examine nicotine dependence.
Biochemical Verification Expired CO was assessed following each training session and at follow-up using a Carbon Monoxide Monitor (Model 3110; Spirometrics, Inc., Auburn, ME).
Approach Bias The approach avoidance task (AAT) in this study was a 15-minute computerized task adapted from Heuer, Rinck, and Becker (2007). Participants were instructed to pull a joystick upon seeing an image tilted to the right and to push the joystick upon seeing a left-tilt image, while ignoring the image content (i.e., indirect task instruction). By pulling the joystick (approach), the picture grew in size; by pushing the joystick away (avoidance), the picture shrunk.
In order to assess the level of approach bias at baseline, participants first completed a total of 96 trials in which each of 24 smoking-related pictures (e.g., woman lighting a cigarette) and each of 24 positive images (e.g., group of friends exercising) were pulled and pushed. The smoking stimuli consisted of pictures of cigarettes or of varied scenes in which individuals were smoking cigarettes. Examples of the positive stimuli include images of positive social interactions or of nature scenes. We selected positive stimuli because there is no intuitive control stimuli to cigarettes (like there is for alcohol; i.e., non-alcoholic beverages) and this set of images has been successfully used in other pilot work by members of our research group (Becker et al. 2016).
An approach bias score for smoking-related pictures was computed for each participant by subtracting the median time it took to pull smoking-related images from the median time it took to push away these images. The reaction time is defined as the time it took the participant to complete the correct full movement (i.e., time it takes to move the joystick in the correct direction and the image disappears from the screen). Therefore, the task did not require error feedback - participants were not able to move on to the next stimuli until the movement was corrected. Accordingly, a positive value indicates an approach tendency toward smoking stimuli, whereas a negative value is indicative of avoidance tendency for smoking images. The bias was also computed for each of the four training sessions. This allowed us to compute an approach bias score at five time points. The task instructions remained the same across all time points. Training sessions are described in detail in the procedure section below.
Smoking Status Self-reports of daily smoking were collected at baseline, throughout the intervention, and at the 1-week follow-up. We employed number of days abstinent (0–7) after the quit attempt as an index of efficacy. Perkins (2014) has argued that this measure is appropriate for indexing smoking cessation in pilot efficacy testing, because (1) the number of days abstinent during the first week of a formal quit attempt predicts quit status at the end of 2-month and 6-month follow-up (Ashare et al. 2013); and (2) quitting within the first 1–2 weeks is predictive of long-term smoking cessation outcomes (Ferguson et al. 2009; Wileyto et al. 2004).
Procedure
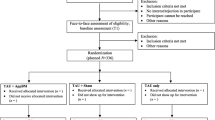
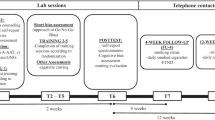
Potential participants completed an online prescreen. Eligible participants were invited to the study site for a baseline visit. Upon arrival, each participant received an informed consent form and a battery of self-report measures. Participants then listened to a brief introduction to the tasks. Participants first completed the baseline approach bias assessment, and then they were randomized to Approach Avoidance Task (AAT) Training or Sham Training. Randomization was stratified based on gender and the severity of nicotine dependence using the FTND (0–4 vs. 5–10). The participants were blind to study condition, but it was not possible to blind staff to study condition in this study as they provided participants with a treatment rationale that was specific to the condition. Study staff instructed participants to make a self-guided quit attempt on the morning following session 4. Participants were told to track their daily cigarette count and were then asked to return to the study site for a one-week follow-up. Participants were compensated for study participation in the form of cash payments ($25) at each visit.
Interventions Each group completed 15 min of training on 4 occasions during a 2-week period. The intervention rationale and instructions were standardized for each group and presented via video message.
AAT Training Participants assigned to the AAT Training condition were told the training may weaken automatic cigarette-approach and strengthen automatic cigarette-avoidance. Using implicit instructions, participants were instructed to pull or push the joystick depending on the tilt of the picture (i.e., right-tilted vs. left-tilted). Each training session comprised 192 training trials, consisting of 96 positive pictures tilted to the right and 96 smoking images tilted to the left. Accordingly, participants in the AAT training condition were trained to avoid almost all smoking-related images and approach almost all positive images. That is, each training session also included an additional 24 “training-incompatible” images distributed evenly across the training trials, where smoking images were tilted to the right and positive images to the left. We used the final 6 incompatible smoking-related trials from the first half of each training session to calculate an approach bias score for each training session (in addition to the baseline score). The bias score was computed for each participant by subtracting the median time it took to pull smoking-related images (the final 6 incompatible trials) from the median time it took to push smoking-related images (96 trials per session). We chose to include the incompatible trials from the first half of the training as this has shown to better capture the learning achieved with AAT training (Loijen et al. 2016). As is the case for the baseline bias scores derived from the AAT, positive values indicate a smoking-approach tendency, whereas negative values indicate a smoking-avoidance tendency.
Sham Training In order to create comparable expectancy effects in both conditions, we also provided participants in the sham training condition with a highly plausible rationale (Eberl et al. 2013; Wiers et al. 2011). They were told that the training would weaken the automatic tendency to approach cigarettes by improving control over this automatic tendency (e.g., learning to ignore urge to approach and respond only to task instructions) and that following the training, they would be easily able to approach or avoid regardless of image content. Participants in the sham condition were instructed to pull or push the joystick depending on the tilt of the picture (i.e., pull right-tilted vs. push left-tilted). Instead of avoiding all smoking-related pictures, however, participants in the sham condition pulled and pushed all pictures equally. This yielded 96 training-compatible trials (48× push smoking, 48× pull positive) and 96 incompatible trials (48× pull smoking, 48x push positive). There were no additional incompatible trials, therefore, the sham training sessions were minimally shorter than the AAT training sessions (192 vs. 216 trials). To compute the approach bias score for each training session among participants assigned to the sham training condition, we used the median response times (RTs) from the 96 smoking-related images (median RT to the 24 incompatible smoking trials minus median RT of the 48 compatible trials). Positive values again indicate a smoking-approach tendency, whereas negative values indicate a smoking-avoidance tendency.
Data Analysis
First, we checked approach bias retraining latencies for treatment integrity. Specifically, trials with errors (i.e., participants pushed when they should have pulled and vice versa) and unusual response latencies (i.e., the lowest and highest 1% of all reaction times) were dropped. Training integrity for a given session was considered unacceptable when 10% or more of the trials were dropped.
Multilevel modeling (MLM) was used to estimate the growth curve for approach bias over time (from baseline to session 4; 5 assessments). MLM is an intent-to-treat analysis that includes all participants, regardless of missing data, thereby increasing power and generalizability. Since approach bias decreased rapidly and then leveled off, we followed the procedure recommended by Heck, Thomas, and Tabata (2013) and others to compare various curvilinear functions of Time (quadratic, logarithmic, hyperbolic) to best fit the data. The model using a hyperbolic function of Time had the best fit. This model showed a fast initial decrease in approach bias, followed by a rapid leveling off.
To test for treatment group differences in reductions in approach bias over time (hypothesis 1), our MLM model included treatment, time (hyperbolic time, centered at end of treatment), and treatment × time as predictors of approach bias. To examine whether baseline approach bias moderated the effect of treatment on change over time (hypothesis 2), we added baseline approach bias, baseline approach bias × treatment, and baseline approach bias × treatment × time to the model. Finally, we performed a regression analysis to determine if the rate of reduction in approach bias predicted length of abstinence after the quit attempt (hypothesis 3). In this analysis, number of days abstinent during the first week after the quit attempt (0–7) was predicted by reduction in approach bias, final approach bias score, treatment condition, baseline CO reading, gender, and age. This analysis was performed using Generalized Least Squares Regression with a log link function and a negative binomial distribution because the dependent variable was count data with overdispersion.
Post-hoc power analyses for the MLM models, performed using the MLM power analysis program PinT 2.12 (Snijders and Bosker 1993), indicated that we had greater than 0.90 power to detect a medium effect size (d = 0.50) for hypotheses 1 and 2. For hypothesis 3, G*Power indicated that we had greater than 0.80 power to detect an effect size of exp(b) = 1.62 for a standardized predictor (i.e., between a medium and a large effect size; Faul et al. 2007).
Results
Sample Characteristics
As can be seen in Table 1, 52 participants were randomized to either AAT (n = 29) or sham (n = 23). Table 1 reports on demographic characteristics and clinical variables assessed at baseline. Overall, there were no between group differences on any demographic or clinical characteristics (all p’s > 0.05). As can be seen in Fig. 1, three participants’ data had to be excluded because their responses on the approach bias assessment were unusually slow. Hence, analyses focusing on approach bias changes over time (hypothesis 1 and 2) included data from 49 individuals. Attendance of training sessions was high (87.8%) with no between-group differences. Of participants that were randomized, 40 returned 1-week later for the follow-up assessment. Accordingly, analyses relating changes in approach bias to days abstinent (hypothesis 3) included data from 40 participants. The mean days abstinent during the first week of the quit attempt was 1.45 (SD = 2.44).
Hypothesis Testing
Consistent with hypothesis 1, the MLM analysis yielded a significant Treatment x Time interaction (b = 159.3, t(47) = 2.65, p = .011, d = 0.77; see Fig. 2). Participants in the AAT condition evidenced a greater decline in approach bias over time than those in the control condition. Hence, participants in AAT had significantly lower approach bias at the end of treatment than those in the control condition (b = 150.7, t(45) = 4.23, p < .001, d = 1.26).
Baseline approach bias did not moderate the effect of treatment on approach bias (hypothesis 2); neither the baseline approach bias x treatment x time interaction nor the baseline approach bias x treatment interaction (at the end of treatment) was significant (ps > 0.41). However, there was a significant interaction between baseline approach bias scores and time (b=-0.98, t(90)=-4.25, p < .001, d = 0.90), indicating that participants with higher baseline approach bias decreased their approach bias more than those with lower baseline approach bias irrespective of condition.
The generalized least squares regression indicated that greater decreases in approach bias were related to a greater number of days abstinent after the quit attempt (b = −0.004, χ 2(1) = 5.45, p = .020, d = 0.79; see Fig. 3.). Baseline CO and sex also emerged as significant predictors such that there were more days abstinent for those with lower CO (b=-0.96, χ 2(1) = 5.57, p = .018, d = 0.80), and for men (b = −0.72, χ 2(1) = 7.17, p = .007, d = 0.93). Finally, neither the level of approach bias at the end of treatment (b = 0.79, χ 2(1) = 2.77, p = .096, d = 0.55), age (b=-0.04, χ 2(1) = 2.10, p = .147, d = 0.47), nor treatment condition (b = −0.50, χ 2(1) = 0.65, p = .420, d = 0.26) were significant predictors of days abstinent. As a sensitivity analysis, we used Expectation Maximization imputation to impute the missing data on days abstinent. Analysis of the imputed number of days abstinent supported our findings from the analysis with the missing data on days abstinent. In particular, greater decreases in approach bias were significantly related to more days abstinent after the quit attempt, (p = .002).
In an exploratory analysis, we found that treatment condition was not significantly related to days abstinent when the slope of improvement in approach bias and the level of approach bias at end of treatment were excluded as predictors of days abstinent in the Poisson regression (b = −0.03, χ 2(1) = 0.01, p = .928, d = 0.25).
Discussion
The current study examined whether four sessions of approach bias modification led to a reduction in approach bias among a sample of motivated treatment-seeking smokers and whether the reduction in approach bias was associated with initial quit success following a self-guided quit attempt. Consistent with study hypotheses, participants assigned to the AAT training condition evidenced significantly greater reduction in approach bias relative to those assigned to the sham training condition. As expected, the AAT training created a smoking-avoidance bias in participants, which decreased from the first to the fourth training session. Also as expected, the sham training condition created a neutral bias by training participants to both pull and push smoking pictures.
Before training, the average approach bias exhibited by all participants was quite small (10 ms). For these motivated smokers, smoking-related stimuli may be ambivalent, evoking both approach and avoidance tendencies. This replicates findings for treatment-seeking, abstinent alcoholics who do not show a strong approach tendency for their drug either (e.g., Eberl et al. 2013). Furthermore, baseline approach bias scores were not correlated with any smoking indices. While this finding was unexpected, it may be that we were analyzing a restricted range of smoking variables; namely, we only analyzed data from smokers.
In our study, individuals with high levels of approach bias at baseline evidenced greater improvement in approach bias over time; however, baseline approach bias did not emerge as a moderator. Our findings suggest that the amount of reduction may be more important than the size of the pre-existing bias when it comes to clinically relevant outcome variables. This finding is inconsistent with some other cognitive bias modification research (Amir et al. 2011), but replicates findings of approach bias modification research in alcoholic inpatients reported by Eberl et al. (2013). It may be that the intervention is most effective when there is a large bias to retrain. Most importantly, a reduction in approach bias during the intervention period was associated with a greater number of days abstinent in the week following a self-guided quit attempt. Collectively, these findings suggest that a brief intervention targeting approach bias may be beneficial in the treatment of smoking cessation, extending the results reported by Machulska et al. (2016) and complementing the literature on approach bias modification in alcohol addiction (Eberl et al. 2013; Wiers et al. 2011).
Previous trials have successfully applied this approach bias retraining as an add-on intervention in inpatient settings (Eberl et al. 2013; Machulska et al. 2016; Wiers et al. 2011). To our knowledge, this is the first laboratory-based test of approach bias modification as a stand-alone intervention among a sample of treatment-seeking smokers. While our brief intervention was effective in reducing the approach bias, the direct effect of treatment condition on abstinence was small (d = 0.25), replicating the small effect on abstinence observed after alcohol-avoidance trainings (Wiers et al. 2011; Eberl et al. 2013). In our study, the effect did not reach statistical significance, possibly because of the relatively small sample in this pilot study. In addition, the sham training may be more effective than assumed. We sought to provide a plausible rationale for the sham group, such that they would be invested in the training (i.e., believing they were improving control over automatic tendencies) but not attuned to explicitly avoid all smoking images as in the AAT training group. However, it may be that 50% smoking-avoidance trials might constitute a weaker dosage of the AAT training, rather than a no-training condition. Thus, while the AAT training should be better at taming the “horse”, the sham training may actually strengthen the “rider”.
Future work should employ the approach bias modification intervention within the context of a dose–response design. Our results suggest a rapid initial decrease in approach bias followed by a leveling off of the bias score. However, there is not much research to lend insight into the optimal number of sessions needed. The general consensus is simply that more than one session is needed (Wiers et al. 2013a). It would also be important to examine mechanisms by which cognitive bias training can improve the quit success. It could be that reducing approach bias helps to alleviate craving or urge to smoke. An additional important next step would be to pair this intervention with a traditional long-term smoking cessation program (Machulska et al. 2016). Such combination approaches are supported by dual process models and empirical evidence (Wiers et al. 2013a).
There are several limitations that warrant consideration. The sample size in the study was relatively small; thus we did not have the power to detect a large effect. Future work should employ this brief intervention among a larger sample. Furthermore, the approach bias score for the AAT group was computed using only the final 6 incompatible trials of the first half of each training session. We chose this strategy in order to measure the score at the optimal stage of learning. However, including a larger number of incompatible trials during the second half of training may have increased the reliability of the bias index. Overall, there is little work examining the reliability of the AAT, and thus, the clinical implications should be interpreted as preliminary. As this was a proof-of-principle study, we excluded individuals who were currently participating in any form of nicotine replacement therapy. Thus, the results may not generalize and future studies should test the approach bias medication among a sample of smokers at differing stages of smoking cessation treatment. While we did observe a relation between approach bias reduction and an index of smoking cessation success, a longer follow-up period is warranted. An additional caveat is that we could be seeing carry-over effects from training to approach positive stimuli, rather than solely training to avoid smoking images. Future studies may employ smoking-matched control stimuli (e.g., an individual holding a pencil to the lips) in order to disentangle these effects.
Another important consideration is that we did not include a post-treatment approach bias assessment. Given the nature of the task design (i.e., pushing and pulling smoking stimuli following the knowledge of the treatment rationale), a final assessment could be therapeutic in itself. We therefore decided to omit this assessment and use the fourth training session as the final assessment point. It is also important to note that we did not do a formal assessment of the training rationale following the intervention; thus, we cannot ascertain that the training rationale for each group was equally plausible. However, each rationale was carefully modeled after the training rationales used in the earlier alcohol trainings (Eberl et al. 2013; Wiers et al. 2011). A final limitation concerns the nature of self-report data—participants may be unwilling to be truthful or lack insight into their daily cigarette count. We sought to ameliorate this risk by telling participants that we were performing biochemical verification of their smoking status, but there are still risks for inaccurate reporting (Man et al. 2009; Shipton et al. 2009; West et al. 2007).
Overall, the current study suggests that a reduction in approach bias may facilitate a smoking cessation success. This lends support to the emergent literature and calls for research testing multi-component, integrative treatments for smoking cessation.
References
Amir, N., Taylor, C. T., & Donohue, M. C. (2011). Predictors of response to an attention modification program in generalized social phobia. Journal of Consulting and Clinical Psychology, 79(4), 533. doi:10.1037/a0023808.
Ashare, R. L., Wileyto, E. P., Perkins, K. A., & Schnoll, R. A. (2013). The first seven days of a quit attempt predicts relapse: Validation of a measure for screening medications for nicotine dependence. Journal of Addiction Medicine, 7(4), 249. doi:10.1097/adm.0b013e31829363e1.
Becker, E. S., Ferentzi, H., Ferrari, G., Möbius, M., Brugman, S., Custers, J., Geurtzen, N., Wouters, J., & Rinck, M. (2016). Always approach the bright side of life: A general positivity training reduces stress vulnerability in vulnerable individuals. Cognitive Therapy and Research, 40, 57–71. doi:10.1007/s10608-015-9716-2.
Cousijn, J., Goudriaan, A. E., & Wiers, R. W. (2011). Reaching out towards cannabis: approach bias in heavy cannabis users predicts changes in cannabis use. Addiction (Abingdon, England), 106(9), 1667–1674. doi:10.1111/j.1360-0443.2011.03475.x.
Eberl, C., Wiers, R. W., Pawelczack, S., Rinck, M., Becker, E. S., & Lindenmeyer, J. (2013). Approach bias modification in alcohol dependence: Do clinical effects replicate and for whom does it work best?Developmental Cognitive Neuroscience ,4, 38–51. doi:10.1016/j.dcn.2012.11.002.
Faul, F., Erdfelder, E., Lang, A. G., & Buchner, A. (2007). G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. doi:10.3758/bf03193146.
Ferguson, S. G., Gitchell, J. G., Shiffman, S., & Sembower, M. A. (2009). Prediction of abstinence at 10 weeks based on smoking status at 2 weeks during a quit attempt: secondary analysis of two parallel, 10-week, randomized, double-blind, placebo-controlled clinical trials of 21-mg nicotine patch in adult smokers. Clinical Therapeutics, 31(9), 1957–1965. doi:10.1016/j.clinthera.2009.08.029.
Field, M., Kiernan, A., Eastwood, B., & Child, R. (2008). Rapid approach responses to alcohol cues in heavy drinkers. Journal of Behavior Therapy and Experimental Psychiatry, 39(3), 209–218. doi:10.1016/j.jbtep.2007.06.001.
Fiore, M. C., Jaen, C. R., Baker, T. B., Bailey, W. C., Benowitz, N., & Curry, S. J. (2008). Treating tobacco use and dependence: 2008 update US Public Health Service Clinical Practice Guideline executive summary. Respiratory Care, 53(9), 1217–1222.
Friese, M., Hofmann, W., & Wiers, R. W. (2011). On taming horses and strengthening riders: Recent developments in research on interventions to improve self-control in health behaviors. Self and Identity, 10(3), 336–351. doi:10.1080/15298868.2010.536417.
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C., & Fagerstrom, K. O. (1991). The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction, 86(9), 1119–1127. doi:10.1111/j.1360-0443.1991.tb01879.x.
Heck, R. H., Thomas, S. L., & Tabata, L. N. (2013). Multilevel and longitudinal modeling with IBM SPSS. Routledge. 10.4324/9780203701249$4.
Heuer, K., Rinck, M., & Becker, E. S. (2007). Avoidance of emotional facial expressions in social anxiety: The Approach–Avoidance Task. Behaviour Research and Therapy, 45(12), 2990–3001. doi:10.1016/j.brat.2007.08.010.
Loijen, A., Rinck, M., Walvoort, S. J. W., Kessels, R. P. C., Becker, E. S., & Egger, J. I. M. (2016). Modification of automatic alcohol approach tendencies in alcohol-dependent patients with mild or major neurocognitive disorder. Manuscript submitted for publication.
Machulska, A., Zlomuzica, A., Adolph, D., Rinck, M., & Margraf, J. (2015). A cigarette a day keeps the goodies away: Smokers show automatic approach tendencies for smoking-, but not for food-related stimuli. PLoS One, 10(2), e0116464. doi:10.1371/journal.pone.0116464.
Machulska, A., Zlomuzica, A., Rinck, M., Assion, H. J., & Margraf, J. (2016). Approach bias modification in inpatient psychiatric smokers. Journal of Psychiatric Research, 76, 44–51. doi:10.1016/j.jpsychires.2015.11.015.
Man, C. N., Fathelrahman, A. I., Harn, G. L., Lajis, R., Samin, A. S. M., Omar, M., & Bayanuddin, N. A. (2009). Correlation between urinary nicotine, cotinine and self-reported smoking status among educated young adults. Environmental Toxicology and Pharmacology, 28(1), 92–96. doi:10.1016/j.etap.2009.03.003.
Perkins, K. A. (2014). Improving efficiency of initial tests for efficacy in smoking cessation drug discovery. Expert Opinion on Drug Discovery,9 (11), 1259–1264. doi:10.1517/17460441.2014.951632.
Shipton, D., Tappin, D. M., Vadiveloo, T., Crossley, J. A., Aitken, D. A., & Chalmers, J. (2009). Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. Bmj, 339, b4347. doi:10.1136/bmj.b4347.
Snijders, T. A., & Bosker, R. J. (1993). Standard errors and sample sizes for two-level research. Journal of Educational and Behavioral Statistics, 18(3), 237–259. doi:10.3102/10769986018003237.
Stacy, A. W., & Wiers, R. W. (2010). Implicit cognition and addiction: a tool for explaining paradoxical behavior. Annual Review of Clinical Psychology, 6, 551. doi:10.1146/annurev.clinpsy.121208.131444.
US Department of Health and Human Services. (2014). The health consequences of smoking—50 years of progress: A report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health,17. doi:10.1037/e510072014-001.
West, R., Zatonski, W., Przewozniak, K., & Jarvis, M. J. (2007). Can we trust national smoking prevalence figures? Discrepancies between biochemically assessed and self-reported smoking rates in three countries. Cancer Epidemiology Biomarkers & Prevention, 16(4), 820–822. doi:10.1158/1055-9965.epi-06-0679.
Wiers, C. E., Kühn, S., Javadi, A. H., Korucuoglu, O., Wiers, R. W., Walter, H., & Bermpohl, F. (2013b). Automatic approach bias towards smoking cues is present in smokers but not in ex-smokers. Psychopharmacology, 229(1), 187–197. doi:10.1007/s00213-013-3098-5.
Wiers, R. W., Eberl, C., Rinck, M., Becker, E. S., & Lindenmeyer, J. (2011). Retraining automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychological Science, 22(4), 490–497. doi:10.1177/0956797611400615.
Wiers, R. W., Gladwin, T. E., Hofmann, W., Salemink, E., & Ridderinkhof, K. R. (2013a). Cognitive bias modification and cognitive control training in addiction and related psychopathology mechanisms, clinical perspectives, and ways forward. Clinical Psychological Science. doi:10.1177/2167702612466547.
Wiers, R. W., Rinck, M., Kordts, R., Houben, K., & Strack, F. (2010). Retraining automatic action - tendencies to approach alcohol in hazardous drinkers. Addiction, 105(2), 279–287. doi:10.1111/j.1360-0443.2009.02775.x.
Wileyto, E. P., Patterson, F., Niaura, R., Epstein, L. H., Brown, R. A., Audrain-McGovern, J., & Lerman, C. (2004). Do small lapses predict relapse to smoking behavior under bupropion treatment?Nicotine & Tobacco Research, 6(2), 357–367. doi:10.1080/1462220042000202463.
Zhou, X., Nonnemaker, J., Sherrill, B., Gilsenan, A. W., Coste, F., & West, R. (2009). Attempts to quit smoking and relapse: factors associated with success or failure from the ATTEMPT cohort study. Addictive Behaviors, 34(4), 365–373. doi:10.1016/j.addbeh.2008.11.013.
Acknowledgements
This study was in part funded by a Grant from the National Institute on Drug Abuse: R34 DA034658-01 (awarded to Dr. Smits)
Author Contribution
SOB, MR, and JAJS designed the randomized controlled trial. SOB, MLD, DR, and JAJS wrote the first draft. DR and MLD conducted the statistical analyses. SOB and JRF collected the data. All authors worked to revise the manuscript and approved the final version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Scarlett O. Baird, Mike Rinck, David Rosenfield, Michelle L. Davis, Jillian R. Fisher, Eni S. Becker, Mark B. Powers, and Jasper A. J. Smits have no conflicts of interest with respect to the research, authorship, and publication of the manuscript.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Animal Rights
This article does not contain any studies with animals performed by any of the authors.
Additional information
Trial Registration ClinicalTrials.gov, NCT02481752, https://clinicaltrials.gov/ct2/show/NCT02481752
Rights and permissions
About this article
Cite this article
Baird, S.O., Rinck, M., Rosenfield, D. et al. Reducing Approach Bias to Achieve Smoking Cessation: A Pilot Randomized Placebo-Controlled Trial. Cogn Ther Res 41, 662–670 (2017). https://doi.org/10.1007/s10608-017-9835-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10608-017-9835-z