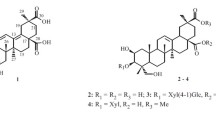
Six lupane triterpenoids (1–6), including an undescribed one, 3,23-di-O-isopropylidene-lup-20(29)-ene (1), along with two oleane triterpenoids (7, 8), were isolated from the Zhuang ethnic medicinal plant Glochidion eriocarpum. Their structures were identified based on comprehensive NMR, MS, and physiochemical data analysis. Compounds 2, 7, and 8 significantly inhibited the production of nitride oxide (NO) of the LPS-induced RAW 264.7 cells, which may be attributed to the anti-inflammatory activity of this plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Inflammation is a complex process mediated by the activation of various immune cells. Many studies have shown that inflammation is associated with various human diseases, including obesity, cancer, asthma, Alzheimer’s disease, diabetes, atherosclerosis, heart failure, and others [1, 2]. Macrophages play a central role in the host defense against foreign agents; once activated by various stimuli, including proinflammatory cytokines and bacterial lipopolysaccharides, they can kill pathogens directly or indirectly via secretion of pro-inflammatory factors [3, 4]. Inhibition of the production of inflammatory cytokines, such as tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), and interleukin 1β (IL-1β), and inflammatory mediators such as nitric oxide (NO) and prostaglandin E2 (PGE2), serves as a key mechanism in the control of inflammation [5].
Glochidion eriocarpum (Champ. ex Benth.) Mull. Arg., a shrub of the family Phyllanthaceae, is known as Anweikun, Meima, and Panmei in the Zhuang ethnic districts of Guangxi, China. The leaves and twigs are widely used for the treatment of rheumatoid arthritis, allergic dermatitis, and detumescence [6]. Previous investigations showed that the chemical constituents of this species are rather simple, mainly triterpenes and their saponins [7,8,9,10], phenolics [11], and flavonoids [12] with cytotoxic activities [8,9,10]. As part of our ongoing search for the active chemical basis from ethnic medicinal plants, a new lupane triterpenoid (1), along with seven known triterpenoids, was isolated from G. eriocarpum. Three of them exhibited significant inhibition of nitric oxide (NO) production in lipopolysaccharide-induced RAW 264.7 cells.
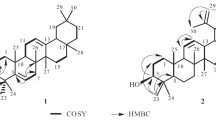
3,23-Di-O-isopropylidene-lup-20(29)-ene (1) was obtained as colorless needles (MeOH). Its molecular formula was established as C33H54O2 by HR-ESI-MS (m/z 483.4192 [M + H]+) (calcd for C33H55O2, 483.4197), indicating 7 indices of hydrogen deficiency. Two olefinic protons at δH 4.69 (1H, d, J = 2.5 Hz), 4.57 (1H, dd, J = 2.5, 1.5 Hz), eight methyl protons at δH 1.68 (s), 1.41 (s), 1.40 (s), 1.04 (s), 0.98 (s), 0.86 (s), 0.79 (s), and 0.69 (s), three protons attached to oxycarbons at δH 3.65 (1H, d, J = 12.0 Hz), 3.61 (1H, t, J = 3.0 Hz), and 3.24 (1H, d, J = 12.0 Hz) in the 1H NMR spectrum, along with 33 carbon signals in its 13C NMR spectrum (Table 1) showed that 1 was a lupane triterpenoid. The chemical shifts of 1 are in good agreement with lup-20(29)ene-3α,23-diol [9], except for the presence of a quaternary carbon at δC 98.2, and two methyls at δC 29.4 and 19.5 in 1. The 1H–1H COSY correlation of H-3 (δ 3.61, t, J = 3.0 Hz) with H-2a (δ 1.89, m) and the HMBC correlations (Fig. 1) of H-32 with C-31 and C-33 and H-33 with C-31 and C-32 suggested the linkage of two methyls C-32 (δ 29.4) and C-33 (δ 19.5) with the quaternary carbon C-31 (δ 98.2), forming an oxygen-bearing isopropyl moiety.
The HMBC correlations of H-3 with C-24, C-5, C-23, and C-31, H-23 with C-31, C-3, C-4, C-5, and C-24 suggested that the quaternary carbon C-31 (δ 98.2) of isopropyl moiety was linked with C-3 and C-23 by two oxygen atoms, forming a new six-membered ring. The configuration of H-3 was identified as β-orientation by the relatively small coupling constant (J = 3.0 Hz) and the NOESY correlations of H-3 with H-24 and H-24 with H-25 (Fig. 1). Thus, the structure of compound 1 was established. Seven known triterpenoids, lup-20(29)ene-3α,23-diol (2) [9], glochidone (3) [9], glochidiol (4) [9], glochidonol (5) [9], dioslupecin A (6) [13], puberoside A (7) [14], and glomeruloside C (8) [15] were also isolated from the plant.
Nontoxicity of the isolated compounds indicated by over 80% cell viability was determined by the MTT colorimetric method. The viability of RAW 264.7 cells improved to different extents when the compound concentration decreased from 50 to 3.125 μM. The RAW 264.7 cells survived more than 80% when the concentrations of compounds 4 and 5 were not greater than 12.5 μM, 25 μM (3, 7, and 8), and 50 μM (1 and 2) respectively. The survival rate was under 80% when the concentration of compound 6 was 3.125 μM.
The IC50 values of the isolated compounds on LPS-stimulated NO production inhibition in RAW 264.7 cells were evaluated (Table 2).
Terpenoids, especially lupane- and oleane-type triterpenoids and their glycosides, were frequently obtained from Glochidion genus. The biological activity evaluation mainly focused on cytotoxic activities. In this study, a lupane and two oleane-type triterpenoids showed significant anti-inflammatory activities.
Experimental
General. The NMR spectra were recorded on a Bruker DRX 500 spectrometer (Bruker Corporation, Switzerland). HR-ESI-MS was performed on a Q-Exactive mass spectrometer (Thermo Fisher Scientific, USA); UV spectrum was obtained using a Mapada UV-1600 spectrophotometer (Mapada, China); Semipreparative HPLC was performed on an Agilent 1260 Liquid Chromatograph System (Agilent Technologies Inc, USA). The positive control indomethacin and LPS were both purchased from Solarbio (Beijing, China). The sulfanilamide and naphthalene ethylenediamine were purchased from Aladdin (Shanghai, China) and Macklin (Shanghai, China) respectively. RAW 264.7 cells were purchased from the Chinese Academy of Sciences Cell Bank (Shanghai, China).
Plant Material. The leaves and twigs of G. eriocarpum (Champ. ex Benth.) Mull. Arg. were collected in 2020 from Hezhou, Guangxi Province, China, and identified by Prof. Yu-Song Huang of Guangxi Institute of Botany, Chinese Academy of Sciences. The voucher specimen (Tge202011) was stored at the Experimental Center of Guilin Medical University.
Extraction and Isolation. The dried leaves and twigs of G. eriocarpum (10.8 kg) were extracted three times with 95% EtOH under reflux at 55°C. The EtOH extracts (588.0 g) were suspended in H2O and successively partitioned with ethyl acetate (EtOAc) and n-BuOH. The EtOAc fraction (169.1 g) was subjected to silica gel column with petroleum ether (PE)–EtOAc (1:0→1:1) and CHCl3–MeOH (15:1→3:1) to yield fractions I–IX. Compound 3 (281.6 mg) was obtained by recrystallization from Fr. II in methanol. Fr. IV was passed through an MCI column (MeOH–H2O, 20:80→98:2) to obtain seven fractions. Fraction IV-6 was fractioned on a silica gel column with PE–EtOAc (8:1→6:1) and recrystallized from CHCl3–MeOH (1:1) to give compound 5 (10.8 mg); Fr. IV-7 was chromatographed on a silica gel column eluting with PE–acetone (40:1→1:1) and RP-18 with MeOH–H2O (90:10), further recrystallized from MeOH, to afford compound 6 (7.7 mg). Fraction V was chromatographed on a silica gel column and eluted with PE–EtOAc (10:1→3:1) to give seven smaller fractions. Fraction V-3 was separated by a silica gel column with PE–EtOAc (30:1→3:1) to give the new compound 1 (15.8 mg); Fr. V-7 was chromatographed on a silica gel column eluting with CH2Cl2–acetone (1:0→3:1) and applied to Sephadex LH-20, eluting with CHCl3–MeOH (1:3, 1:6, 0:1), further separated on a silica gel column using stepwise gradient elution with PE–EtOAc (8:1→2:1), and purified through RP-18 using MeOH–H2O (98:2) as an elution to obtain compounds 4 (26.0 mg) and 2 (15.3 mg). The n-BuOH (138.6 g) extract was combined and crudely separated on a D101 column, eluting with EtOH–H2O (10:90→95:5) to give five fractions (Frs. A–E). Fraction E was applied to a silica gel column using a gradient system of increasing polarity with CHCl3–MeOH (8:1→1:1) to yield eight fractions (Frs. E-1–8). Fraction E-5 was separated by RP-18 eluting with MeOH–H2O (75:25→90:10) and followed by preparative HPLC to obtain compound 7 (134.9 mg, ACN–H2O + 0.1% TFA 55:45, tR = 106.949 min). Fraction E-6 was passed through a Sephadex LH-20 column (MeOH) followed by silica gel column chromatography with CHCl3–MeOH (4:1→3:1), and was further purified by preparative HPLC to afford compound 8 (43.3 mg, MeOH–H2O, 80:20, tR = 34.031 min).
3,23-Di-O-isopropylidene-lup-20(29)-ene (1), colorless needles (MeOH), mp 118–120°C, [α]20D +17.0° (c 1.0, MeOH). UV (MeOH, λmax, nm): 202. IR (KBr, ν, cm–1): 2970, 2893, 1647, 1454, 1381, 1088. (+)-HR-ESI-MS m/z 483.4192 [M + H]+ (calcd for C33H55O2, 483.4197). For 1H (500 MHz, CDCl3) and 13C NMR (125 MHz, CDCl3) data, see Table 1.
Bioassay of Cell Viability and NO Production Inhibition were determined according to the method described in Chen et al. [16], and indomethacin was used as a positive control.
Statistical Analysis. Statistical analysis was performed by independent t test using SPSS 26.0 software. Results are shown as means ± SD and expressed as IC50 values.
References
M. Kacirova, A. Zmeskalova, L. Korinkova, B. Zelezna, J. Kunes, and L. Maletinska, Clin. Sci., 134, 547 (2020).
T. Suetomi, S. Miyamoto, and J. H. Brown, Am. J. Physiol. Heart Circ. Physiol., 317, H877 (2019).
Y. R. Mahida, Inflamm. Bowel Dis., 6, 21 (2000).
M. E. Bianchi and A. A. Manfredi, Proc. Natl. Acad. Sci. USA, 111, 2866 (2014).
X. F. Wang, H. Li, K. Jiang, Q. Q. Wang, Y. H. Zheng, W. Tang, and C. H. Tan, Fitoterapia, 130, 61 (2018).
M. R. Jia and X. W. Li, Summary of Chinese Ethnic Medicine [in Chinese], China Medical Science and Technology Press, Beijing, 2005, 300 pp.
W. H. Hui and M. M. Li, Phytochemistry, 15, 561 (1976).
P. Van Kiem, V. K. Thu, P. H. Yen, N. X. Nhiem, N. H. Tung, N. X. Cuong, C. V. Minh, H. T. Huong, J. H. Hyun, H. K. Kang, and Y. H. Kim, Chem. Pharm. Bull., 57, 102 (2009).
P. Puapairoj, W. Naengchomnong, A. Kijjoa, M. M. Pinto, M. Pedro, M. S. J. Nascimento, A. M. S. Silva, and W. Herz, Planta Med., 71, 208 (2005).
N. X. Nhiem, V. K. Thu, P. Van Kiem, C. Van Minh, B. H. Tai, T. H. Quang, N. C. Cuong, P. H. Yen, H. J. Boo, J. I. Kang, H. K. Kang, and Y. H. Kim, Arch. Pharmacol. Res., 35, 19 (2012).
Y. Wang, H. Zhu, D. Wang, R. Cheng, C. Yang, M. Xu, and Y. Zhang, Bull. Korean Chem. Soc., 35, 631 (2014).
V. K. Thu, P. Van Kiem, C. Van Minh, P. H. Yen, N. X. Cuong, and H. J. Huong, J. Chem., 48, 125 (2010).
Y. H. Kuo, C. I. Chang, S. Y. Li, C. J. Chou, C. F. Chen, Y. H. Kuo, and K. H. Lee, Planta Med., 63, 363 (1997).
Z. Zhang, Z. L. Gao, X. Fang, Y. H. Wang, H. Xiao, X. J. Hao, G. M. Liu, and H. P. He, J. Asian Nat. Prod. Res., 10, 1029 (2008).
V. K. Thu, N. V. Thang, N. X. Nhiem, B. H. Tai, N. H. Nam, P. V. Kiem, C. V. Minh, H. L. T. Anh, N. Kim, S. Park, and S. H. Kim, Phytochemistry, 116, 213 (2015).
J. Chen, D. L. Li, L. N. Xie, Y. R. Ma, P. P. Wu, C. Li, W. F. Liu, K. Zhang, R. P. Zhou, X. T. Xu, X. Zheng, and X. Liu, Phytomedicine, 78, 153309 (2020).
Acknowledgment
This work was financially supported by the National Natural Science Foundation of China (82060783), Natural Science Foundation of Guangxi (2023GXNSFAA026017) and the Fund of the State Key Laboratory of Phytochemistry and Plant Resources in West China (P2022-KF13).
In addition, Jin-Ni Tan and Chun-Hua Lai contributed equally to this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 4, July–August, 2024, pp. 563–565.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tan, JN., Lai, CH., Wei, W. et al. Triterpenoids from Glochidion eriocarpum. Chem Nat Compd 60, 649–652 (2024). https://doi.org/10.1007/s10600-024-04405-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-024-04405-4