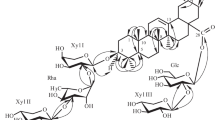
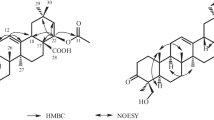
A new oleanane triterpene saponin 1 was isolated from Ligularia veitchiana. The structure of compound 1 was elucidated as olean-12-en-6β,16β,28-triol 3-O-β-D-glucuronopyranoside by spectroscopic data (1H, 13C NMR, HMBC, HSQC, COSY, and NOSEY) and chemical evidence. Additionally, compound 1 presented phytotoxic effect on the growth of lettuce at 200 ppm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ligularia veitchiana (Hemsl.) Greenm., a well-known traditional herbal medicine, is grown in northwestern China [1]. It has been used in traditional Chinese medicine to treat influenza, cough, ulcer, and pulmonary tuberculosis [2]. Previous chemical studies of this plant have reported the isolation of several compounds including sesquiterpenes, triterpenes, neolignans, phenylpropanoids, benzofurans, and their derivatives [2,3,4,5]. Among them, some isolated compounds showed a variety of bioactivities, such as anti-inflammatory, anticancer, and nematocidal activities [4,5,6]. To find new active compounds, the chemical constituents of L. veitchiana were investigated. From the n-butanol-soluble fraction, we have isolated a new oleanane triterpene saponin (1). In this paper, we describe the isolation, structural elucidation, and phytotoxic effect of the new triterpene saponin.
Compound 1 was obtained as a yellow gum, was found to have the molecular formula C36H58O10 by the quasi-molecular ion peak at m/z 651.4106 ([M + H]+ calcd 651.4108) in the HR-ESI-MS, indicating eight degrees of unsaturation. Its IR spectrum showed absorption bands at 3348, 1708, and 1650 cm–1 owing to hydroxy, carboxy, and olefine functionalities. Acid hydrolysis of 1 revealed one unit of glucuronic acid (identified by co-TLC with the authentic sample and detailed studies of COSY, HSQC, and HMBC spectra) [7]. The 1H NMR spectrum of 1 showed signals for seven tertiary methyl groups at δ 0.88, 0.92, 1.12, 1.13, 1.18, 1.23, and 1.28 (each 3H, s, H3-29, 30, 25, 23, 27, 24, 26), three methines bearing oxygen function [δ 3.09 (1H, dd, J = 9.6, 5.5 Hz, H-3), 4.49 (1H, br.s, H-6), and 4.22 (1H, dd, J = 11.8, 4.5 Hz, H-16)], one methylene bearing oxygen function [δ 3.78 (1H, d, J = 9.5 Hz) and 3.25 (1H, m), H2-28], and one olefinic proton at δ 5.25 (1H, br.s, H-12). The 13C NMR spectrum showed signals corresponding to 36 carbons, including a carboxy carbon at δ 175.5 (C-6′), two olefinic carbons at δ 143.7 (C-13) and 124.2 (C-12), four oxygenated carbons at δ 91.1 (C-3), 68.6 (C-6), 67.9 (C-16), and 68.9 (C-28), and seven tertiary methyl carbons at δ 28.4 (C-23), 18.5 (C-24), 17.5 (C-25), 18.7 (C-26), 27.5 (C-27), 31.7 (C-29), and 24.3 (C-30). Furthermore, the spectrum also showed a signal at δC 105.6 (C-1′) assignable to an anomeric carbon in a sugar unit. These above proton and carbon data suggested that 1 might be assignable to an oleanane-type triterpene skeleton with a sugar moiety. Its aglycone was determined to be olean-12-en-3β,6β,16β,28-tetraol [8], owing to their similar NMR data, except for the presence of one set of resonances attributable to a D-glucuronopyranosyl moiety [H-1′ (δH 4.37, 1H, d, J = 8.0 Hz), δC 105.6, 75.4, 78.1, 74.1, 76.5, and 175.5] in 1 [9]. The β-anomeric configuration of the sugar unit was deduced from the coupling constant (8.0 Hz). In the HMBC experiment, the correlation between H-3 and C-1′ indicated the attachment of glucuronic acid at C-3 (Fig. 1). The three hydroxyl groups in 1 were assigned on C-6, C-16, and C-28, respectively, owing to the COSY correlations of H-5–H-6–H-7 and H-15–H-16 (Fig. 1), as well as HMBC correlations of H-6 to C-5/C-6/C-7, H-16 to C-15/C-16/C-17, and H2-28 to C-16/C-17/C-22 (Fig. 1). The relative configuration of 1 was confirmed by NOESY (Fig. 1). The α-orientation of H-3 and H-6 was deduced from the correlations of H-3 with H-5, H-6, and H3-23 and the α-orientation of H-16 was deduced from the correlation of H-16 and H3-27. Based on the above analysis, the structure of compound 1 (Fig. 1) was established as olean-12-en-6β,16β,28-triol 3-O-β-D-glucuronopyranoside.
The phytotoxic effect of compound 1 on lettuce growth was examined. Compound 1 inhibited the growth of root (66.4%) and hypocotyl (68.9%) of lettuce at 200 ppm.
Experimental
General Experimental Procedures. IR spectrum (neat or KBr) was recorded on a Perkin Elmer 2000 FT-IR spectrometer. HR-ESI-MS was recorded on a Bruker APEX II mass spectrometer. NMR spectra of the new compound were recorded on a Bruker Avance 600 MHz spectrometer with tetramethylsilane (TMS) as internal standard. Silica gel (200–300 mesh; Qingdao Marine Chemical Factory, China) and Sephadex LH-20 (40–70 μm; GE Healthcare, USA) were used for column chromatography (CC).
Plant Material. The plants of L. veitchiana were collected in Zhangjiajie, Hunan Province, People’s Republic of China, in September, 2018, and identified by Hai-bo Wu. A voucher specimen (No. 20180906) was deposited at Minzu University of China.
Extraction and Isolation. The air-dried and powdered whole herbs of L. veitchiana (1.6 kg) were extracted at reflux three times with 95% ethanol, for 4 h each. The filtered and concentrated extract was dissolved in water and then sequentially partitioned with petroleum ether, chloroform, and n-butanol to yield four portions. The n-butanol fraction (80 g) was subjected to repeated CC using chloroform–methanol (50:1, 25:1, 10:1, 5:1, and 2:1) as a solvent system to produce five fractions (F1–F5). Fraction F3 was subjected to Sephadex LH-20 CC (chloroform–methanol, 1:1) and then was separated by silica gel CC using chloroform–methanol, 8:1 to afford 1 (2.3 mg).
Olean-12-en-6 β ,16 β ,28-triol 3- O - β -D-glucuronopyranoside (1), yellow gum, \( {\left[\upalpha \right]}_{\textrm{D}}^{20} \) –19.8° (c 0.1, MeOH). IR (νmax, cm–1): 3348, 1708, 1650. HR-ESI-MS m/z 651.4106 [M + H]+ (calcd for C36H59O10, 651.4108). 1H NMR (600 MHz, CD3OD, δ, ppm, J/Hz): 5.25 (1H, br.s, H-12), 4.49 (1H, br.s, H-6), 4.37 (1H, d, J = 8.0, H-1′), 4.22 (1H, dd, J = 11.8, 4.5, H-16), 3.78 (1H, d, J = 9.5, H-28a), 3.61 (1H, d, J = 9.9, H-5′), 3.45 (1H, t, J = 9.1, H-4′), 3.38 (1H, t, J = 9.0, H-3′), 3.25 (1H, m, H-28b), 3.21 (1H, t, J = 7.9, H-2′), 3.09 (1H, dd, J = 9.6, 5.5, H-3), 2.16 (1H, dd, J = 13.1, 3.3, H-18), 2.15 (1H, m, H-22a), 1.99 (1H, m, H-11a), 1.88 (1H, m, H-11b), 1.79 (1H, m, H-2a), 1.73 (1H, m, H-7a), 1.73 (1H, m, H-19a), 1.73 (1H, m, H-2b), 1.57 (1H, dd, J = 10.4, 7.1, H-9), 1.55 (1H, m, H-1a), 1.55 (1H, m, H-7b), 1.41 (1H, m, H-21a), 1.41 (1H, m, H-22b), 1.38 (1H, d, J = 13.4, H-15a), 1.28 (3H, s, H-26), 1.23 (3H, s, H-24), 1.21 (1H, m, H-21b), 1.18 (3H, s, H-27), 1.18 (1H, d, J = 12.8, H-15b), 1.13 (3H, s, H-23), 1.12 (3H, s, H-25), 1.00 (H, d, J = 13.1, H-19b), 0.94 (1H, m, H-1b), 0.92 (3H, s, H-30),0.88 (3H, s, H-29), 0.77 (1H, br.d, J = 10.8, H-5). 13C NMR (150 MHz, CD3OD, δ, ppm): 42.0 (C-1), 27.1 (C-2), 91.1 (C-3), 41.1 (C-4), 57.3 (C-5), 68.6 (C-6), 41.5 (C-7), 40.4 (C-8), 48.5 (C-9), 37.2 (C-10), 24.6 (C-11), 124.2 (C-12), 143.7 (C-13), 45.1 (C-14), 34.8 (C-15), 67.9 (C-16), 40.9 (C-17), 45.0 (C-18), 47.8 (C-19), 31.5 (C-20), 34.7 (C-21), 25.8 (C-22), 28.4 (C-23), 18.5 (C-24), 17.5 (C-25), 18.7 (C-26), 27.5 (C-27), 68.9 (C-28), 31.7 (C-29), 24.3 (C-30), 105.6 (C-1′), 75.4 (C-2′), 78.1 (C-3′), 74.1 (C-4′), 76.5 (C-5′), 175.5 (C-6′).
References
Q. Liu, L. Shen, T. T. Wang, C. J. Chen, W. Y. Qi, and K. Gao, Food Chem., 122, 55 (2010).
D. Xu, H. Z. Song, J. L. Xu, W. M. Hu, X. Y. Fan, H. Wang, and K. Zou, Nat. Prod. Res., 36, 701 (2022).
C. F. Wang, Y. Zhao, Y. Z. Liu, and Z. Z. Zhang, Helv. Chim. Acta, 91, 1712 (2008).
H. Zhu and P. Tu, Z. Naturforsch. B., 59, 1063 (2004).
Y. Zhao, H. Peng, and Z. Jia, J. Nat. Prod., 57, 1626 (1994).
H. B. Wu, T. T. Liu, W. J. Deng, J. Zhang, and L. W. Yang, Chem. Nat. Compd., 55, 671 (2019).
J. L. Yang, T. K. Q. Ha, B. Dhodary, E. Pyo, N. H. Nguyen, H. Cho, E. Kim, and W. K. Oh, J. Med. Chem., 58, 1268 (2015).
W. S. Wang, K. Gao, C. M. Wang, and Z. J. Jia, Pharmazie, 58, 148 (2003).
H. B. Wu, T. T. Liu, W. S. Wang, J. C. Feng, and H. M. Tian, Molecules, 22, 1981 (2017).
Acknowledgment
This work was financially supported by the National Natural Science Foundation of China [32000238 and 31800278] and Young Teachers Scientific Research Ability Improvement Program of Minzu University of China [2022QNPY58].
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2022, pp. 909–910.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, Px., Wu, Hb. A New Triterpene Saponin from Ligularia veitchiana. Chem Nat Compd 58, 1079–1081 (2022). https://doi.org/10.1007/s10600-022-03871-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-022-03871-y