One new compound, β-D-apiofuranosyl-(1→6)-1-ethyl-O-β-D-glucopyranoside (1), and 11 known compounds were isolated from Trichosanthis Radix. Their structures were mainly elucidated by NMR and MS spectra. All the isolated compounds were tested for their inhibitory activities against α-glucosidase, and compound 11 exhibited potential inhibitory activity with an IC50 value of 36.41 ± 0.54 μM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Trichosanthis Radix is the dry roots of Trichosanthes kirilowii Maxim. or Trichosanthes rosthornii Harms, which is an important traditional Chinese medicine for treating diabetes and fever. Modern biological studies have demonstrated that this herb has hypoglycemic [1], anti-inflammatory [2], antiherpes virus [3], and antitumor activity [4]. Previous phytochemical studies showed that this herb was rich in trichosanthin [5], lectins [6], polysaccharide [7], triterpenes [8], and amino acid [9]. The reported hypoglycemic components of Trichosanthis Radix are trichosans A, B, C, D, and E [10] and lectin [11]. In this paper, the isolation of one new compound and 11 known compounds from the water fraction of Trichosanthis Radix and their α-glucosidase inhibitory activities are reported.
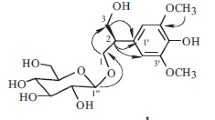
Compound 1 was isolated as a yellow liquid. It was found to possess the molecular formula C13H24O10 by HR-ESI-MS (m/z 375.1061 [M + Cl]–), which was confirmed by 13C and DEPT NMR spectra. The 13C NMR spectra of compound 1 showed the presence of one CH3, four CH2, and seven CH, as well as one quaternary carbon atom.
Based on the comparison of compound 1 with the literature, the 1H NMR and 13C NMR spectra of 1 (Table 1) were similar to those of methyl β-D-apiofuranosyl-(1→6)-β-D-glucopyranoside [12]. The major differences between compound 1 and methyl β-D-apiofuranosyl-(1→6)-β-D-glucopyranoside were the new signals of δC 67.2 (C-1), 16.4 (C-2), and the disappearance of δC 56.7 (OCH3) in compound 1. In the 1H–1H COSY spectrum, the correlations of δ 1.27 (3H, t, J = 7.2 Hz, H-2) with δ 3.96 (1H, m, H-1α) and δ 3.64 (1H, m, H-1β) suggested the presence of [CH2CH3]. In the HMBC spectrum, the correlations of δH 3.96 (1H, m, H-1α) and δH 3.64 (1H, m, H-1β) with δC 105.0 (C-1′), and the correlations of δ 3.64 (1H, m, H-1β) with δC 77.7 (C-5′) indicated that the ethyl was connected to C-1′. Thus, the structure of 1 was established as β-D-apiofuranosyl-(1→6)-1-ethyl-O-β-D-glucopyranoside.
Careful analysis of the 1D NMR and MS spectral data resulted in the identification of the other 11 known compounds as methyl-α-D-fructofuranoside (2) [13], 3-(β-D-ribofuranosyl)-2,3-dihydro-6H-1,3-oxazine-2,6-dione (3) [14], uridin (4) [15], uracil (5) [16], allantoin (6) [17], 2,2′-(leucyl azanediyl)-bis-(3-methylpentanoic acid) (7) [18], L-phenylalaninamide (8) [19], N-methyl-2-pyrrolidinone (9) [20], tyrosine (10) [21], 3,3′,5,5′-tetra-tert-butyl-2,2′-dihydroxybiphenyl (11) [22], and 5α,6β-dihydroxy daucosterol (12) [23].
Except for compounds 5 and 8, all the other 10 compounds were isolated from this plant to our knowledge for the first time. In our investigation, it was found that compound 11 exhibited potential inhibitory activity (IC50 = 36.41 ± 0.54 μM) against α-glucosidase (positive control acarbose IC50 = 0.17 ± 0.0036 μM), and it might be developed as an α-glucosidase inhibitory candidate to control the blood sugar level of diabetics.

Experimental
General. NMR spectra were recorded on a Bruker-Avance III-600 MHz spectrometer with TMS as internal standard. MS data were obtained on a MS Waters AutoSpec Premier P776 mass spectrometer. HR-ESI-MS data were acquired using a UPLC-IT-TOF MS spectrometer. UV data were obtained using a UV-2700 series spectrophotometer. IR data were recorded on a NiCOLET iS10 spectrophotometer. Optical rotation data were obtained using a Autopol VI spectrometer.
Plant Material. The dried roots of Trichosanthes kirilowii Maxim, Trichosanthis Radix, were purchased from Hele Chinese Medicine Co., Ltd., in August, 2019, and identified by Dr. Yumei Zhang from Xishuangbanna Tropical Botanical Garden, Chinese Academy of Science. A voucher specimen (No. 20190815) for Trichosanthis Radix was deposited in the Innovative Drug Research Group of Xishuangbanna Tropical Botanical Garden.
Extraction and Isolation. The air-dried and powdered roots of T. kirilowii (20 kg) were extracted with 90% methanol and filtered at room temperature. The filtrate was concentrated and extracted with petroleum ether, then extracted by ethyl acetate, BuOH, and H2O. The water fraction (514.9 g) was subjected to silica gel column chromatography and eluted with CHCl3–MeOH (20:1–0:1) to generate six fractions (Frs. 1–6). Fraction 1 (16.1 g) was subjected to silica gel column chromatography and eluted with CHCl3–MeOH (30:1–0:1), Sephadex LH-20 column chromatography and eluted with CHCl3–MeOH (1:1) to yield compounds 5 (28.0 mg), 4 (8.9 mg), and 12 (5.1 mg). Fraction 2 (18.8 g) was subjected to silica gel column chromatography and eluted with CHCl3–MeOH (30:1–0:1) and repeatedly crystallized to yield compound 8 (7.2 mg). Fraction 3 (7.2 g) was separated by Sephadex LH-20 with MeOH–CHCl3 (1:1) and a BUCHI RP-C18 medium pressure column with MeOH–H2O (10:90) to yield compound 1 (4.0 mg). Fraction 4 (14.7 g) was subjected to silica gel column chromatography and eluted with petroleum ether–ethyl acetate (10:1–1:1) and ethyl acetate–MeOH (20:1–1:1) to yield compounds 6 (3.6 mg), 9 (2.8 mg), 3 (2.3 mg), and 11 (2.8 mg). Fraction 5 (77.1 g) was subjected to silica gel column chromatography and eluted with CHCl3–MeOH (15:1–1:1) and repeatedly crystallized to yield compounds 7 (31.0 mg) and 10 (61.0 mg). Fraction 6 (44.0 g) was further chromatographed over a BUCHI RP-C18 medium-pressure column with MeOH–H2O (10:90–95:5) to yield compound 2 (4.3 mg).
β -D-Apiofuranosyl-(1→6)-1-ethyl-O-β-D-glucopyranoside (1), C13H24O10, yellow liquid, \( {\left[\upalpha \right]}_{\mathrm{D}}^{24.8} \) –26.1° (c 0.105, DMSO). UV/Vis (MeOH, λmax, nm) (log ε): 196 (3.61). IR (KBr, ν, cm–1): 3407, 2928, 2886, 1638, 1406, 1054, 1023, 577. For 1H and 13C NMR data, see Table 1. HR-ESI-MS m/z 375.1061 [M + Cl]– (calcd 375.1063).
α -Glucosidase Inhibitory Activities. The sample to be tested (final concentration 20 μg/mL), enzyme solution (final concentration 0.025 U/mL), buffer solution and substrate (final concentration 1 mM) were sequentially added to the 96-well cell culture plates, fully mixed, and repeated with two wells. At the same time, the blank control and acarbose (final concentration 1 μg/mL) positive control were set up. Incubated at 37°C for 50 min, the optical density value at 405 nm was measured by an enzyme labeling instrument, and the inhibition rates of α-glucosidase activities were calculated.
References
X. M. Zhang, X. L. Niu, N. N. Wei, C. L. Xia, Q. F. Zhang, S. Y. Gao, S. J. Guo, and D. H. Yang, Chin. J. Ethnomed. Ethnopharm., 29, 13 (2020).
X. R. Zhou, N. Yang, L. M. Lu, Q. Ding, Z. J. Jiao, Y. Zhou, and K. Y. Chou, Immunol. Lett., 110, 74 (2007).
G. F. Chen, F. Yin, H. Y. Zhang, W. G. Huang, and F. Y. Chen, Chin. J. Clin., Electron Ed, 4, 1520 (2010).
F. Wang, X. Y. Deng, M. H. Yao, T. Tian, Y. Liu, H. Wang, J. Wang, and X. Y. Dong, China Med. Her., 16, 13 (2019).
N. Pushpa, K. M. Nai, B. L. Phuong, and N. S. W. Ricky, Plant Sci., 162, 79 (2002).
Q. Li, X. L. Ye, H. Zeng, X. Chen, and X. G. Li, J. Chin. Med. Mater., 35, 475 (2012).
X. L. Niu, K. P. Ji, J. J. Zhong, N. N. Wei, and L. P. Peng, Guizhou Agric. Sci., 42, 164 (2014).
T. H. Manh, N. P. Thanh, A. K. Jeong, K. O. Won, H. L. Jeong, H. W. Mi, and S. M. Byung, Bioorg. Chem., 83, 105 (2019).
D. F. Gong, F. C. Wang, D. H. Ji, and H. X. Qin, Chin. Tradit. Pat. Med., 41, 607 (2019).
H. Hikino, M. Yoshizawa, and Y. Suzuki, Planta Med., 55, 349 (1989).
L. Ming, C. J. Ji, and W. P. Yao, Protein Pept. Lett., 8, 81 (2001).
T. Tomomi, I. Toru, and K. Junichi, Phytochemistry, 63, 479 (2003).
X. J. Shi, J. H. Zhu, L. Yang, and R. M. Yu, Chin. Tradit. Herb. Drugs, 42, 870 (2011).
Y. F. Jiang, H. G. Choi, Y. Li, Y. M. Park, J. H. Lee, D. H. Kim, J. Lee, J. K. Son, M. K. Na, and S. H. Lee, Arch. Pharm. Res., 34, 2021 (2011).
B. Wang, P. Liu, Y. P. Shen, and C. Dai, China J. Chin. Mater. Med., 30, 895 (2005).
Y. H. Zhu, X. S. Huang, W. C. Ye, and G. X. Zhou, Chin. Pharm. J., 47, 1029 (2012).
T. J. Ma, P. F. Tu, F. J. Lv, and X. S. Hu, Acta Bot. Boreali-Occident Sin., 26, 1732 (2006).
A. A. Dissanayake, B. A. H. Ameen, and M. G. Nair, J. Nat. Prod., 9, 2472 (2017).
H. L. Ge and J. G. Dai, China J. Chin. Mater. Med., 35, 3151 (2010).
D. Xu, R. S. Jin, and H. X. Wang, J. Trop. Subtrop. Bot., 27, 102 (2019).
N. Lv, L. G. Shen, G. Z. Li, J. C. Wang, and J. Y. Si, Mod. Chin. Med., 19, 488 (2017).
Y. B. Liu, X. R. Cheng, J. J. Qin, S. K. Yan, H. Z. Jin, and W. D. Zhang, Chin. J. Nat. Med., 9, 0115 (2011).
S. H. Li, H. J. Zhang, P. Yao, X. M. Niu, W. Xiang, and H. D. Sun, J. Asian Nat. Prod. Res., 4, 147 (2002).
Acknowledgment
This investigation was supported by grants from the Strategic Pilot Program A of the Chinese Academy of Sciences (Grant No. XDA12040219), the Institute of Drug Innovation of the Chinese Academy of Sciences (Grant No. CASIMM0520181002), and the Science and Technology Major Project of Yunnan Province under Grant (202102AA100014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2022, pp. 674–676.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, XL., Zhang, K., Ni, JY. et al. α-Glucosidase Inhibitory Constituents from Trichosanthis Radix. Chem Nat Compd 58, 796–798 (2022). https://doi.org/10.1007/s10600-022-03800-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-022-03800-z