A novel biphenyl derivative, insularione (3,3′,4,4′,6-pentahydroxy-3,4-dihydro-[1,1′-biphenyl]-2,5-dione) (1), was isolated from the stems of Cinnamomum insulari-montanum Hayata (Lauraceae) along with six known compounds. The structure of the novel biphenyl derivative was characterized on the basis of spectral analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cinnamomum insulari-montanum Hayata (Lauraceae) is an endemic tree that grows in Taiwan′s natural hardwood forest at elevations between 400 and 1500 m [1]. Previously, we isolated 16 compounds, including two butanolides, three lignans, two flavonoids, one coumarin, four steroids, and four benzenoids from this plant [2, 3]. In the course of screening for bioactive components from Formosan plants in the family Lauraceae [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26], C. insulari-montanum was chosen for further phytochemical investigation. The chemical constituents in the stems of C. insulari-montanum Hayata were separated with column chromatography. A novel biphenyl derivative, insularione (3,3′,4,4′,6-pentahydroxy-3,4-dihydro-[1,1′-biphenyl]-2,5-dione) (1), along with eight known compounds, including a mixture of β-sitosterol (2) and stigmasterol (3) [27], coumarin (4) [28], cinnamyl alcohol (5) [28], cinnamic acid (6) [28], p-hydroxybenzoic acid (7) [28], kaempferol (8) [29], and kaempferitrin (9) [29], were isolated from C. insulari-montanum Hayata. In this paper, we report the isolation and structural elucidation of the novel compound.
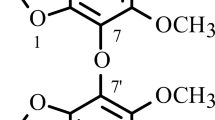
Insularione (3,3′,4,4′,6-pentahydroxy-3,4-dihydro-[1,1′-biphenyl]-2,5-dione) (1) was obtained as a white amorphous powder from MeOH. Its molecular formula was deduced as C12H10O7 by HR-ESI-MS m/z 289.0322 [M + Na]+ (calcd 289.0324). The IR spectrum of 1 showed characteristic absorption bands due to the presence of the hydroxyl (3300 cm–1) and carbonyl (1680 cm–1) moieties. The 1H NMR spectrum of 1 displayed the typical pattern of an ABX type at δ 7.41 (1H, d, J = 8.4 Hz, H-5′), 8.10 (1H, dd, J = 8.4, 2.0 Hz, H-6′), and 8.62 (1H, d, J = 2.0 Hz, H-2′) and two methines deshielded by two conjugated carbonyls appearing at δ 6.74/6.78 (each 1H, d, J = 2.0 Hz, H-4/3), indicating that 1 was probably a trisubstituted benzene. The 13C NMR and DEPT experiments of 1 showed 12 resonance signals consisting of five methines and seven quaternary carbons. The structure 1 was also confirmed by 2D NMR experiments (Fig. 1). A COSY correlation was observed between the H-3 and H-4 and between the H-5′ and H-6′ (Fig. 1). The HETCOR experiment showed that the carbon signals at δ 94.2 for C-4, 99.2 for C-3, 116.6 for C-2′, 116.6 for C-5′, and 121.0 for C-6′ were correlated to the proton signals at δ 6.74 for H-4, δ 6.78 for H-3, δ 8.62 for H-2′, δ 7.41 for H-5′, and δ 8.10 for H-6′, respectively.
The relative configuration of 1 was assigned by analysis of the 1H NMR coupling constants and NOESY experiment. The mutual gauche junction was deduced from the observation of the quasi-1,2-diequatorial interactions of H-3 and H-4, and the coupling constants were 2.0 Hz. Due to the small quantity of isolated material and instability of compound 1, the absolute configuration of C-3 & 4 remains to be determined. Thus, 1 is a novel biphenyl derivative, which was further confirmed by COSY, HMBC, and NOESY experiments.
Experimental
General. UV spectra were obtained in MeCN, and IR spectra were measured on a Hitachi 260-30 spectrophotometer. 1H NMR (400 MHz, CD3OD) and NOESY spectra were obtained on a Varian (Unity Plus) NMR spectrometer. Low-resolution ESI-MS spectra were obtained on an API 3000 (Applied Biosystems), and high-resolution ESI-MS spectra were recorded on a Bruker Daltonics APEX II 30e spectrometer. Silica gel 60 (Merck, 70–230 mesh, 230–400 mesh) was used for column chromatography. Precoated silica gel plates (Merck, Kieselgel 60 F-254), 0.20 and 0.50 mm, were used for analytical TLC and preparative TLC, respectively, visualized with 50% H2SO4.
Plant Material. The specimen of C. insulari-montanum Hayata was collected from Pingtung County, Taiwan, March 2003. A voucher specimen (Cinnamo. 3) was identified by Dr. Fu-Yuan Lu (Department of Forestry and Natural Resources College of Agriculture, National Chiayi University) and was deposited in the School of Medical and Health Science, the Fooyin University, Kaohsiung, Taiwan.
Extraction and Isolation. The stems (7.0 kg) of C. insulari-montanum Hayata were extracted repeatedly with MeOH at room temperature for 24–48 h. The MeOH extract was dried and evaporated to leave a viscous residue (82.4 g). The residue was placed on a silica gel column and eluted with CHCl3 gradually enriched with MeOH to afford 17 fractions (Frs. 1–17). Part of Fr. 3 (12.5 g) was rechromatographed on silica gel (n-hexane–EtOAc, 18:1) and recrystallized from EtOAc to give coumarin (4) and a mixture of β-sitosterol (2) and stigmasterol (3) (201 mg). Part of Fr. 6 (26.3 g), eluted from n-hexane–EtOAc (1:2), was further chromatographed on silica gel elution with EtOAc–MeOH (15:1) and recrystallized from acetone to give cinnamic acid (6) (49 mg) and cinnamyl alcohol (5) (51 mg), respectively. Part of Fr. 11 (7.6 g) was purified by silica gel chromatography (EtOAc–MeOH, 10:1) to give colorless needles of kaempferol (8) (25 mg). Part of Fr. 14 (5.2 g) was purified by silica gel chromatography (EtOAc–MeOH, 8:1) to give colorless needles of p-hydroxybenzoic acid (7) (52 mg). Part of Fr. 16 (4.2 g) was subjected to silica gel chromatography by eluting with EtOAc–MeOH (4:1) and enriched with MeOH to furnish four further fractions (16-1–16-4). Fractions 16-3 (1.2 g) eluted with EtOAc–MeOH (4:1) was further purified using silica gel column chromatography and the same solvent system to give insularione (3,3′,4,4′,6-pentahydroxy-3,4-dihydro-[1,1′-biphenyl]-2,5-dione) (1) (9 mg). Part of Fr. 17 (13.6 g) was rechromatographed on silica gel (EtOAc–MeOH, 5:1) and recrystallized from MeOH to give kaempferitrin (9) (781 mg).
Insularione (1), white amorphous powder. UV (MeCN, λmax, nm) (log ε): 397 (3.11). IR (νmax, cm–1): 3300 (br, OH), 1680 (C=O). ESI-MS m/z 289 [M + Na]+; HR-ESI-MS m/z 289.0322 [M + Na]+ (calcd for C12H10O7Na, 289.0324). 1H NMR (400 MHz, C5D5N, δ, ppm, J/Hz): 6.74 (1H, d, J = 2.0, H-4), 6.78 (1H, d, J = 2.0, H-3), 7.41 (1H, d, J = 8.4, H-5′), 8.10 (1H, dd, J = 8.4, 2.0, H-6′), 8.62 (1H, d, J = 2.0, H-2′). 13C NMR (100 MHz, C5D5N, δ, ppm): 94.2 (C-4, CH), 99.2 (C-3, CH), 116.6 (C-2′, CH), 116.6 (C-5′, CH), 121.0 (C-6′, CH), 137.9 (C-1′, C), 147.1 (C-3′, C), 147.7 (C-4′, C), 157.4 (C-1, C), 162.4 (C-6, C), 165.5 (C-5, C=O), 177.2 (C-2, C=O).
β -Sitosterol (2) and stigmasterol (3) as in [27], white needles (CH2Cl2), mp 138–140°C. IR (νmax, cm–1): 3400, 2910, 1620, 1450.
Coumarin (4) as in [28], colorless oil. UV (MeCN, λmax, nm): 260, 330. IR (νmax, cm–1): 1730, 1630. 1H NMR (400 MHz, CDCl3, δ, ppm, J/Hz): 6.43 (1H, d, J = 9.5, H-3), 7.29 (1H, td, J = 8.0, 1.2, H-6), 7.34 (1H, dd, J = 8.0, 1.2, H-5), 7.50 (1H, dd, J = 8.0, 1.2, H-8), 7.54 (1H, td, J = 8.0, 1.2, H-7), 7.72 (1H, d, J = 9.5, H-4).
Cinnamyl alcohol (5) as in [28], yellowish needles (CH2Cl2), mp 208–210°C. UV (MeCN, λmax, nm): 260, 315. IR (νmax, cm–1): 1700, 1680, 1650, 980, 770. 1H NMR (400 MHz, CDCl3, δ, ppm, J/Hz): 4.28 (2H, d, J = 5.6, H-1), 6.15 (1H, dt, J = 16.0, 5.6, H-2), 6.47 (1H, d, J = 16.0, H-3), 7.38 (3H, m, H-6, 7, 8), 7.58 (2H, m, H-5, 9).
Cinnamic acid (6) as in [28], yellowish needles (CH2Cl2), mp 208–210°C. 1H NMR (400 MHz, CDCl3, δ, ppm, J/Hz): 6.63 (1H, d, J = 16.0, H-2), 7.38 (3H, m, H-6, 7, 8), 7.56 (1H, d, J = 16.0, H-3), 7.58 (2H, m, H-5, 9).
p -Hydroxybenzoic acid (7) as in [28], brown powder (CH2Cl2). 1H NMR (400 MHz, CDCl3, δ, ppm, J/Hz): 6.85 (2H, d, J = 8.6, H-3, 5), 7.97 (2H, d, J = 8.6, H-2, 6).
Kaempferol (8) as in [29], yellow needles (MeOH), mp 275–277°C. 1H NMR (400 MHz, CD3OD, δ, ppm, J/Hz): 6.25 (1H, d, J = 2.0, H-6), 6.57 (1H, d, J = 2.0, H-8), 6.74 (2H, d, J = 8.8, H-3′, 5′), 7.38 (2H, d, J = 8.8, H-2′, 6′).
Kaempferitrin (9) as in [29], yellow amorphous powder. UV (MeCN, λmax, nm): 220, 265, 360. IR (νmax, cm–1): 3400, 1680, 1620, 1560. 1H NMR (400 MHz, CD3OD, δ, ppm, J/Hz): 0.81 (3H, d, J = 5.6, 3-O-Rha-CH3), 1.11 (3H, d, J = 6.4, 7-O-Rha-CH3), 5.30 (1H, d, J = 1.6, 3-O-Rha-1′′), 5.56 (1H, d, J = 1.6, 7-O-Rha-1′′), 6.42 (1H, d, J = 2.4, H-6), 6.75 (1H, d, J = 2.4, H-8), 6.90 (2H, d, J = 8.8, H-3′, 5′), 7.75 (2H, d, J = 8.8, H-2′, 6′).
References
T. W. Hu, Y. T. Lin, and C. K. Ho, Bull. Taiwan For. Res. Inst. Eng., 78, 18 (1985).
T. J. Hsieh, S. F. Hsieh, and C. Y. Chen, Chem. Nat. Compd., 46, 99 (2010).
C. Y. Chen, C. L. Lin, C. L. Kao, C. T. Chen, and H. T. Li, Chem. Nat. Compd., 55, 922 (2019).
J. Li, C. Y. Chen, J. Y. Huang, L. Wang, Z. Xu, W. Kang, M. H. Lin, and H. M. Wang, Oxid. Med. Cell. Longev., 2020, 1 (2020).
R. J. Lin, C. L. Kao, S. L. Liu, H. C. Yeh, P. L. Song, H. T. Li, H. M. Wang, and H. W. Chang, Chem. Nat. Compd., 56, 604 (2020).
C. Y. Chen, C. M. Liu, H. M. Wu, H. C. Yeh, W. J. Li, H. T. Li, and H. W. Chang, Chem. Nat. Compd., 56, 621 (2020).
C. T. Chen, C. L. Kao, W. J. Li, H. C. Yeh, H. T. Li, and C. Y. Chen, Chem. Nat. Compd., 55, 1185 (2019).
C. Y. Chen, C. L. Kao, W. J. Li, H. C. Yeh, S. C. Huang, and H. T. Li, Chem. Nat. Compd., 54, 562 (2018).
C. L. Lin, M. H. Perng, W. J. Li, H. T. Li, and C. Y. Chen, Chem. Nat. Compd., 54, 628 (2018).
Z. L. Hong, J. C. Huang, S. Y. Kuo, and C. Y. Chen, Nat. Prod. Commun., 6, 1297 (2011).
C. Y. Chen, Z. L. Hong, W. L. Yang, M. H. Wu, and J. C. Huang, Nat. Prod. Res., 26, 1218 (2012).
C. Y. Chen and Y. D. Wang, Chem. Nat. Compd., 47, 215 (2011).
C. Y. Chen, Chem. Nat. Compd., 47, 306 (2011).
Y. C. Chia, H. C. Yeh, Y. T. Yeh, and C. Y. Chen, Chem. Nat. Compd., 47, 220 (2011).
I. J. Lin, H. C. Yeh, T. M. Cham, and C. Y. Chen, Chem. Nat. Compd., 47, 43 (2011).
T. J. Hsieh, S. F. Hsieh, and C. Y. Chen, Chem. Nat. Compd., 46, 99 (2010).
C. Y. Chen, H. M. Wang, S. H. Chung, W. L. Lo, W. L. Yang, and S. C. Yang, Chem. Nat. Compd., 46, 474 (2010).
C. Y. Chen, W. L. Yang, and Y. R. Hsui, Nat. Prod. Res., 24, 423 (2010).
M. J. Cheng, W. L. Lo, W. S. Tseng, H. C. Yeh, and C. Y. Chen, Nat. Prod. Res., 24, 732 (2010).
I. J. Lin, W. L. Lo, Y. C. Chia, L. Y. Huang, T. M. Cham, W. S. Tseng, Y. T. Yeh, H. C. Yeh, Y. D. Wang, and C. Y. Chen, Nat. Prod. Res., 24, 775 (2010).
R. J. Lin, M. J. Cheng, J. C. Huang, W. L. Lo, Y. T. Yeh, C. M. Teh, C. M. Lu, and C. Y. Chen, J. Nat. Prod., 72, 1816 (2009).
C. Y. Chen, C. H. Chen, Y. C. Lo, B. N. Wu, H. M. Wang, W. L. Lo, C. M. Yen, and R. J. Lin, J. Nat. Prod., 71, 933 (2008).
S. Y. Kuo, T. J. Hsieh, Y. D. Wang, W. L. Lo, Y. R. Hsui, and C. Y. Chen, Chem. Pharm. Bull., 56, 97 (2008).
C. Y. Chen, C. H. Chen, C. H. Wong, Y. W. Liu, Y. S. Lin, Y. D. Wang, and Y. R. Hsui, J. Nat. Prod., 70, 103 (2007).
C. H. Chen, W. L. Lo, Y. C. Liu, and C. Y. Chen, J. Nat. Prod., 69, 927 (2006).
T. J. Hsieh, C. H. Chen, W. L. Lo, and C. Y. Chen, Nat. Prod. Commun., 1, 21 (2006).
T. J. Hsieh, Y. C. Wu, S. C. Chen, C. S. Huang, and C. Y. Chen, J. Chin. Chem. Soc., 51, 869 (2004).
T. J. Hsieh, C. C. Su, C. Y. Chen, C. H. Liou, and L. H. Lu, J. Mol. Struct., 741, 193 (2005).
C. Y. Chen, S. L. Hsieh, M. M. Hsieh, S. F. Hsieh, and T. J. Hsieh, Chin. Pharm. J., 56, 141 (2004).
Acknowledgment
This investigation was supported by a Grant from the Fooyin University.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2022, pp. 20–22.
Rights and permissions
About this article
Cite this article
Chen, C.Y., Wu, M.D., Yeh, H.C. et al. A Novel Biphenyl Derivative from Cinnamomum insulari-montanum. Chem Nat Compd 58, 18–20 (2022). https://doi.org/10.1007/s10600-022-03590-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-022-03590-4