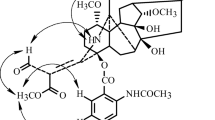
A series of new lappaconitine structural analogs were prepared via esterification of the lappaconine C-4 hydroxyl by aromatic acids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Lappaconitine is an available diterpene alkaloid with a rather broad spectrum of biological activity and the design basis of the antiarrhythmic drug allapinine [1]. The activity of allapinine is largely determined by the C-4 ester incorporating an anthranilic acid. The preparation of lappaconitine analogs with various aromatic acids in the ester has been reported. As a rule, these compounds were obtained by modifying the anthranilic moiety [2,3,4,5,6,7,8,9,10,11,12,13,14].
One possible pathway to introducing an ester is to esterify lappaconine (1) at the C-4 alcohol with aromatic acids.
Three tertiary alcohols in the 4-, 8-, and 9-positions occur in 1. Acylation is known to be more difficult for tertiary than primary and secondary alcohols. However, the C-4 OH of 1 was shown to be slightly more easily acylated than the 8- and 9-OH groups [15].

The carbodiimide method was used by us to activate the acid and acylate 1 at the C-4 OH group with aromatic acids because esterification of the tertiary alcohol was difficult.
Esterification of 1 by picolinic acid occurred in anhydrous CH2Cl2 under Ar with a reagent ratio of lappaconine–dicyclohexylcarbodiimide (DCC)–dimethylaminopyridine (DMAP)–acid of 1:2:0.5:1.3 with heating (40°C) for 6 h and storage for 1 d at room temperature. Ester 2 was obtained in 47% yield based on 45% conversion of 1.
The APCI mass spectrum of 2 gave m/z 529 [M + H]+. IR spectroscopy indicated that 2 was an ester (ν 1714 cm–1). The structure of 2 was established by NMR spectroscopy using two-dimensional COSY, HSQC, HMBC, and NOESY correlation methods (Table 1).
13C NMR spectra of 2 recorded in DEPT 90 and DEPT 135 modes and with full suppression of protons determined unambiguously the chemical shifts (CSs) of 7 methylene, 12 methine, 4 methyl, and 6 quaternary C atoms. The CSs of the protons corresponding to them were found in HSQC spectra.
Compound 2, like 1, contained three methoxyls and an EtN group according to the PMR spectrum. Its 13C NMR spectrum showed four doublets and a singlet in the δC 120–150 ppm region of CSs characteristic of aromatic C atoms for a Py fragment (δ 124.8 d, 126.5 d, 136.8 d, 149.0 s, and 149.9 d); its PMR spectrum, resonances for the four aromatic protons corresponding to them in (δ 7.40 t, 7.75 t, 7.95 d, and 8.70 d). This indicated that the alcohol was esterified. The C-4 resonance of 2 (δ 84.4 ppm) was shifted to weak field as compared to the literature for 1 (δ 71.1 ppm) [16], indicating that the C-4 OH was esterified.
Esters of 1 with nicotinic (3), p-nitro- (4), m-nitrobenzoic (5), and pyromucic (furan-2-carboxylic) acids were analogously obtained in moderate yields (56, 58, 72, and 74%, respectively, based on conversion of 1). However, esterification of 1 with 2-benzyloxybenzoic acid to prepare the salicylate was unsuccessful, as was an attempt at esterification with 2-methoxybenzoic acid.
Resonances of C atoms (Table 1) and the protons corresponding to them were fully assigned using series of 2D NMR spectral experiments for synthesized 2–6. PMR and 13C NMR spectra of all synthesized compounds agreed with their structures and contained sets of resonances characteristic of the diterpene part of the skeleton and the corresponding substituents.
Experimental
IR spectra were recorded from films on a Shimadzu Prestige 21 FTIR spectrophotometer in the range 4000–400 cm–1. Chemical ionization at atmospheric pressure (APCI) mass spectra were obtained on an LC-MS-2010 EV quadrupole LC-MS (Shimadzu). PMR and 13C NMR spectra were recorded in CDCl3 with TMS internal standard on a Bruker Avance III-500 spectrometer (500.13 MHz for 1H, 125.76 MHz for 13C). Resonances in PMR and 13C NMR spectra were accurately assigned using COSY, HSQC, HMBC, NOESY, 13C DEPT 135 and DEPT 90 methods for recording NMR spectra embedded in the spectrometer operating system and full suppression of protons. CSs of protons were determined from 2D HSQC spectra. The spectral part of the research was conducted on equipment at the Khimiya CUC, UIC, UFRC, RAS and the Agidel′ RCUC, UFRC, RAS.
Lappaconine (1) was prepared by alkaline hydrolysis of lappaconitine [17].
General Method for Preparing Esters 2–6. A solution of esterifying acid (1.3 mmol) in dry CH2Cl2 (12 mL) under Ar was stirred, treated with DCC (0.412 g, 2 mmol) in dry CH2Cl2 (10 mL), held at room temperature for 20 min, stirred, treated dropwise with a solution of 1 (0.423 g, 1 mmol) in dry CH2Cl2 (10 mL) with added DMAP (0.061 g, 0.5 mmol), stirred with heating (40°C) under Ar for 6 h, and left for 1 d at room temperature. The precipitated N,N′-dicyclohexylurea was separated. The solvent was distilled off. The solid was separated by column chromatography (CC) over SiO2 using C6H6–MeOH.
Aconitane-4,8,9-triol-1,14,16-trimethoxy-20-ethyl-4-picolinate (2). Elution by C6H6–MeOH (1.5%) afforded ester 2 (0.111 g, 21% yield, 47% based on conversion of 1); by C6H6–MeOH (3%), starting lappaconine (1, 0.234 g, 45% conversion). IR spectrum (κBr, ν, cm–1): 3400 (OH), 1714 (OC=O). Mass spectrum (APCI), m/z 529 [M + H]+. 1H NMR spectrum (δ, ppm, J/Hz): 1.08 (3H, t, J = 7.2, NCH2CH3), 1.59 (1H, dd, J = 15.0, 8.2, Ha-6), 1.86 (1H, m, Ha-3), 1.96 (1H, m, Ha-12), 2.00 (1H, dd, J = 15.0, 7.6, Ha-15), 2.08 (1H, dd, J = 12.5, 4.5, H-10), 2.13 (1H, d, J = 7.9, H-7), 2.15 (1H, m, Ha-2), 2.28 (1H, m, Hb-2), 2.34 (1H, m, H-13), 2.37 (1H, m, Hb-15), 2.47 (1H, m, H-5), 2.48 (1H, m, NCHaCH3), 2.49 (1H, m, Hb-12), 2.54 (1H, m, NCHbCH3), 2.55 (1H, d, J = 11.4, Ha-19), 2.65 (1H, m, Hb-3), 2.66 (1H, m, Hb-6), 2.98 (1H, s, H-17), 3.18 (1H, dd, J = 10.3, 7.0, H-1), 3.27 (3H, s, 1-OCH3), 3.28 (1H, m, H-16), 3.28 (3H, s, 16-OCH3), 3.37 (3H, s, 14-OCH3), 3.42 (1H, d, J = 4.7, H-14), 3.62 (1H, d, J = 11.4, Hb-19), 7.40 (1H, t, J = 7.0, H-4′), 7.75 (1H, t, J = 7.8, H-5′), 7.95 (1H, d, J = 7.8, H-6′), 8.71 (1H, d, J = 4.0, H-3′).
Aconitane-4,8,9-triol-1,14,16-trimethoxy-20-ethyl-4-nicotinate (3). Elution by C6H6–MeOH (1.5%) afforded ester 3 (0.141 g, 27% yield, 56% based on conversion of 1); by C6H6–MeOH (3%), starting lappaconine (1, 0.223 g, 47% conversion). IR spectrum (κBr, ν, cm–1): 3400 (OH), 1716 (OC=O). Mass spectrum (APCI), m/z 529 [M + H]+. 1H NMR spectrum (δ, ppm, J/Hz): 1.13 (3H, t, J = 7.1, NCH2CH3), 1.59 (1H, dd, J = 14.9, 8.2, Ha-6), 1.82 (1H, m, Ha-3), 2.02 (1H, m, Ha-12), 2.04 (1H, m, Ha-15), 2.11 (1H, m, H-10), 2.17 (1H, m, H-7), 2.18 (1H, m, Ha-2), 2.30 (1H, m, Hb-2), 2.38 (1H, m, H-13), 2.39 (1H, m, Hb-15), 2.44 (1H, m, H-5), 2.47 (1H, m, Hb-12), 2.58 (1H, m, NCHaCH3), 2.59 (1H, d, J = 11.4, Ha-19), 2.60 (1H, m, NCHbCH3), 2.70 (1H, m, Hb-3), 2.72 (1H, m, Hb-6), 3.02 (1H, s, H-17), 3.21 (1H, m, H-1), 3.30 (1H, m, H-16 and 3H, s, 1-OCH3), 3.31 (3H, s, 16-OCH3), 3.39 (3H, s, 14-OCH3), 3.44 (1H, d, J = 4.4, H-14), 3.63 (1H, d, J = 11.4, Hb-19), 3.65 (1H, s, 9-OH), 7.34 (1H, t, J = 7.8, H-5′), 8.18 (1H, d, J = 7.8, H-6′), 8.73 (1H, br.s, H-4′), 9.12 (1H, s, H-2′).
Aconitane-4,8,9-triol-1,14,16-trimethoxy-20-ethyl-4- p -nitrobenzoate (4). Elution by C6H6–MeOH (1.5%) afforded ester 4 (0.157 g, 27% yield, 58% based on conversion of 1); by C6H6–MeOH (3%), lappaconine (1, 0.222 g, 48% conversion). IR spectrum (κBr, ν, cm–1): 3400 (OH), 1722 (OC=O). Mass spectrum (APCI), m/z 573 [M + H]+. 1H NMR spectrum (δ, ppm, J/Hz): 1.16 (3H, t, J = 7.1, NCH2CH3), 1.60 (1H, dd, J = 15.1, 8.2, Ha-6), 1.86 (1H, m, Ha-3), 2.02 (1H, m, Ha-12), 2.05 (1H, dd, J = 15.0, 7.8, Ha-15), 2.14 (1H, m, H-10), 2.15 (1H, m, Ha-2), 2.19 (1H, d, J = 7.9, H-7), 2.28 (1H, m, Hb-2), 2.38 (1H, m, Hb-12), 2.39 (1H, m, Hb-15), 2.40 (1H, m, H-13), 2.48 (1H, d, J = 7.2, H-5), 2.61 (1H, d, J = 11.3, Ha-19), 2.65 (1H, m, NCHaCH3), 2.67 (1H, m, NCHbCH3), 2.70 (1H, m, Hb-3), 2.72 (1H, dd, J = 15.1, 7.8, Hb-6), 3.08 (1H, s, H-17), 3.25 (1H, dd, J = 9.2, 6.6, H-1), 3.31 (6H, s, 1-OCH3, 16-OCH3), 3.32 (1H, m, H-16), 3.40 (3H, s, 14-OCH3), 3.45 (1H, d, J = 4.5, H-14), 3.73 (1H, d, J = 11.3, Hb-19), 8.09 (2H, d, J = 8.7, H-2′, 6′), 8.23 (2H, d, J = 8.7, H-3′, 5′).
Aconitane-4,8,9-triol-1,14,16-trimethoxy-20-ethyl-4- m -nitrobenzoate (5). Elution by C6H6–MeOH (1.5%) afforded ester 5 (0.252 g, 44% yield, 72% based on conversion of 1); by C6H6–MeOH (3%), starting lappaconine (1, 0.166 g, 61% conversion). IR spectrum (κBr, ν, cm–1): 3400 (OH), 1721 (OC=O). Mass spectrum (APCI), m/z 573 [M + H]+. 1H NMR spectrum (δ, ppm, J/Hz): 1.11 (3H, t, J = 7.1, NCH2CH3), 1.61 (1H, m, Ha-6), 1.92 (1H, m, Ha-3), 2.02 (1H, dd, J = 15.0, 7.8, Ha-15), 2.03 (1H, m, Ha-12), 2.10 (1H, m, H-10), 2.16 (1H, m, Ha-2), 2.17 (1H, d, J = 7.9, H-7), 2.29 (1H, m, Hb-2), 2.38 (1H, m, H-13), 2.39 (1H, m, Hb-12), 2.40 (1H, m, Hb-15), 2.47 (1H, m, H-5), 2.60 (1H, d, J = 11.4, Ha-19), 2.64 (1H, m, NCHaCH3), 2.66 (1H, m, NCHbCH3), 2.68 (1H, m, Hb-3), 2.70 (1H, dd, J = 15.1, 7.7, Hb-6), 3.00 (1H, s, H-17), 3.19 (1H, dd, J = 9.8, 6.9, H-1), 3.29 (3H, s, 1-OCH3), 3.30 (3H, s, 16-OCH3), 3.32 (1H, m, H-16), 3.40 (3H, s, 14-OCH3), 3.45 (1H, d, J = 4.5, H-14), 3.60 (1H, d, J = 11.4, Hb-19), 7.60 (1H, t, J = 7.7, H-5′), 8.25 (1H, d, J = 7.7, H-6′), 8.37 (1H, d, H-4′), 8.72 (1H, s, H-2′).
Aconitane-4,8,9-triol-1,14,16-trimethoxy-20-ethyl-4-(furan-2-carboxylate) (6). Elution by C6H6–MeOH (1.5%) afforded ester 6 (0.265 g, 51% yield, 74% based on conversion of 1); by C6H6–MeOH (3%), starting lappaconine (1, 0.130 g, 69% conversion). IR spectrum (κBr, ν, cm–1): 3400 (OH), 1716 (OC=O). Mass spectrum (APCI), m/z 518 [M + H]+. 1H NMR spectrum (δ, ppm, J/Hz): 1.04 (3H, t, J = 7.1, NCH2CH3), 1.52 (1H, dd, J = 15.0, 8.2, Ha-6), 1.76 (1H, m, Ha-3), 1.92 (1H, m, Ha-12), 2.00 (1H, m, Ha-15), 2.02 (1H, m, H-10), 2.08 (1H, d, J = 8.0, H-7), 2.09 (1H, m, Ha-2), 2.22 (1H, m, Hb-2), 2.30 (2H, m, H-5, 13), 2.31 (1H, m, Hb-15), 2.42 (1H, m, NCHaCH3), 2.44 (1H, m, Hb-12), 2.47 (1H, d, J = 11.4, Ha-19), 2.49 (1H, m, NCHbCH3), 2.57 (1H, m, Hb-3), 2.61 (1H, m, Hb-6), 2.94 (1H, s, H-17), 3.12 (1H, dd, J = 10.2, 6.9, H-1), 3.23 (3H, s, 1-OCH3), 3.25 (3H, s, 16-OCH3), 3.26 (1H, m, H-16), 3.34 (3H, s, 14-OCH3), 3.38 (1H, d, J = 4.4, H-14), 3.50 (1H, d, J = 11.4, Hb-19), 6.40 (1H, t, J = 1.9, H-4′), 6.99 (1H, d, J = 3.3, H-3′), 7.47 (1H, br.s, H-5′).
References
S. F. Sokolov, Vestn. Aritmol., 64, 60 (2011).
S. A. Ross and S. W. Pelletier, Heterocycles, 32 (7), 1307 (1991).
T. M. Gabbasov, E. M. Tsyrlina, and S. G. Yunusova, Chem. Nat. Compd., 54, 947 (2018).
T. M. Gabbasov, E. M. Tsyrlina, L. V. Spirikhin, and M. S. Yunusov, Chem. Nat. Compd., 54, 951 (2018).
V. E. Romanov, E. E. Shul’ts, M. M. Shakirov, and G. A. Tolstikov, Chem. Nat. Compd., 44, 346 (2008).
V. E. Romanov, Candidate Dissertation in Chemical Sciences, N. N. Vorozhtsov Inst. Org. Chem., SB, RAS, Novosibirsk, 2008, 146 pp.
Q. H. Chen and F. P. Wang, Chin. Chem. Lett., 12 (5), 421 (2001).
S. A. Osadchii, Doctoral Dissertation in Chemical Sciences, N. N. Vorozhtsov Inst. Org. Chem., SB, RAS, Novosibirsk, 2008, 220 pp.
S. A. Osadchii, E. E. Shul’ts, E. V. Polukhina, M. M. Shakirov, and G. A. Tolstikov, Russ. Chem. Bull., 6, 1077 (2006).
V. E. Romanov, E. E. Shul’ts, M. M. Shakirov, and G. A. Tolstikov, Chem. Nat. Compd., 46, 593 (2010).
N. V. Malykhina, S. A. Osadchii, M. M. Shakirov, E. E. Shul’ts, and G. A. Tolstikov, Dokl. Chem., 394 (1–3), 16 (2004).
A. A. Stepanov, S. F. Vasilevsky, and G. A. Tolstikov, Chem. Sustainable Develop., 18, 425 (2010).
S. A. Osadchii, E. E. Shul’ts, S. F. Vasilevskii, E. V. Polukhina, A. A. Stepanov, and G. A Tolstikov, Russ. Chem. Bull., 56 (2), 356 (2007).
S. F. Vasilevskii, S. A. Osadchii, E. E. Shul’ts, E. V. Polukhina, A. A. Stepanov, and G. A. Tolstikov, Dokl. Chem., 415, 4(2), 181 (2007).
M. Khaimova, N. Mollov, P. Cemeva, A. Antonova, and V. Ivanova, Tetrahedron Lett., 38, 2711 (1964).
F. P. Wang, Q. H. Chen, and X. T. Liang, The C18-Diterpenoid Alkaloids, in: The Alkaloids, G. A. Cordell (ed.), Vol. 67, Elsevier Science, New York, 2009, pp. 1–78.
L. Marion, L. Fonzes, C. K. Wilkins, J. P. Boca, F. Sandberg, R. Thorsen, and E. Linden, Can. J. Chem., 45, 969 (1967).
Acknowledgment
The work was financially supported by RSF Grant No. 19-13-00096.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2021, pp. 437–439.
Rights and permissions
About this article
Cite this article
Gabbasov, T.M., Tsyrlina, E.M., Komissarova, N.G. et al. Esterification of Lappaconine by Aromatic Acids. Chem Nat Compd 57, 512–515 (2021). https://doi.org/10.1007/s10600-021-03399-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-021-03399-7