A new cyclohexanone derivative (stipularone) was isolated from the leaves of Bridelia stipularis (L.) Blume and characterized using 1D and 2D NMR spectroscopy (PMR, 13C NMR, HSQC, DEPT-135, COSY, and HMBC) and HR-ESI-MS. Four known compounds were also identified, 3-(4′-hydroxyphenyl)-2-propenoic acid (4′′-carboxyl)-phenyl ester, β-amyrin, scopoletin, and 4-hydroxybenzoic acid, by comparing their 1H NMR spectra with published results. The chemotaxonomic significance was discussed based on the structure of the isolated compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Bridelia Willd. is a genus of the family Phyllanthaceae, the second-largest segregate of a large and economically important family Euphorbiaceae [1,2,3]. The genus consists of approximately 60–70 species from Africa to Asia [4]. Bridelia stipularis (L.) Blume is a climbing shrub, which grows in shady and moist forest floors and is found in the forest areas of Bangladesh, India, and Myanmar [5]. The plant is locally called Harinhara. It is also known as Pat Khowi [5] and Bangari gach [6].
Several traditional uses have been reported from this plant. For instance, the leaf juice was found to treat allergy in the Chakma community of Chittagong Hill Tracts [6]. A combination of Bridelia stipularis and Curcuma longa was used as folklore medicine in India to treat jaundice [7]. Antidiabetic activity was also reported for the ethanolic leaf extract in streptozotocin-induced diabetic rats [8]. The bark decoction was reported to be used in traditional medicine for the treatment of asthma, intestinal worms, and cough, while the leaves were used against colic [4].
A recent study on the methanol extract of stem bark shows the presence of bioactive steroid and triterpenoids identified as glut-5(6)-en-3-one, glut-5(6)-en-3α-ol, and (22E)-7-hydroxy-28-methylcholesta-4,22-dien-3-one [5]. Another review showed that a fatty alcohol C22H46O, named bridelyl alcohol, and a phlobatanin were isolated from the leaves. Furthermore, taraxenone was separated from the hexane extract of roots [4].
In this paper, we report the isolation and identification of a new cyclohexanone derivative as well as four known compounds from Bridelia stipularis and their chemotaxonomy.

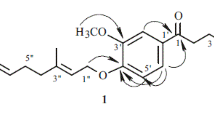
Compound 1 was isolated as a colorless liquid. Accurate mass measurement indicated the empirical formula C8H10O3 (found [M + H]+ at m/z 155.0697 and [M – H]– at m/z 153.0559) from the HR-ESI-MS. The 13C NMR spectrum displayed eight carbon resonances, while the HSQC experiment confirmed that six out of the eight carbons were attached to protons. The DEPT-135 confirmed the presence of three methylene groups and three methine or methyl groups. The PMR spectrum showed two olefinic proton resonances at δ 6.03 and 6.78 ppm, assignable to protons H-6 and H-7 respectively, as confirmed by the COSY signals. The double-doublets at δ 4.09 and 3.96 ppm (J = 14.8, 8.4 Hz) indicate two non-equivalent oxymethylene protons H-3, and the multiplets at δ 2.36 and 2.26 ppm are attributed to non-equivalent protons H-4. The relationship between the proton signals of H-3 and H-4 was confirmed by the COSY signals. The proton signal at δ 4.25 ppm, assignable to the oxymethine proton H-1, displayed a triple-doublet, which can be attributed to the coupling with non-equivalent protons (H-9) at δ 2.79 and 2.62 ppm. The 13C NMR resonance at δ 196.81 ppm is characteristic for a ketone group. Based on these spectral features, a previously isolated cyclohexanone derivative reported in 1973 from Digitalis purpurea [9] seems to match with our findings, but that study lacked 2D NMR data. If we consider the HMBC spectrum, the prominent spot at δH 2.26/2.36 → δC 147.86 (H-4→C-7) cannot be explained in the case of the previously reported structure. Henceforth, on the basis of the complete NMR data presented in Table 1, the structure of compound 1 was deduced as 5-hydroxy-2-oxabicyclo-(3,3,1)-non-6-en-8-one. This new cyclohexanone derivative was given the trivial name stipularone.
The structure of the other compounds was identified by comparing the PMR data with previously published results. These are 3-(4′-hydroxyphenyl)-2-propenoic acid (4′′-carboxyl)-phenyl ester (2) [10], β-amyrin (3) [11], scopoletin (4) [12], and 4-hydroxybenzoic acid (5) [13], respectively.
Compound (1) is a new compound and is very similar to another cyclohexanone derivative isolated from Digitalis purpurea that was reported by Raymakers and Compernolle [9]. The authors of that report reasonably assumed that there might be a close relationship between this type of cyclohexanone derivatives and the biosynthetic pathway of digitoxose. Thus, it is very much possible that there might be chemotaxonomic relations between the family of Digitalis purpurea (Plantaginaceae) and that of Bridelia stipularis (Phyllanthaceae). Also, this is only the second time a cyclohexanone derivative was isolated from a natural source.
Compounds 2–5 have been identified for the first time from Bridelia stipularis. Among them, compound 3 was reported from B. tomentosa [14], and in that report it was claimed that β-amyrin was isolated for the first time from the genus Bridelia. Therefore, to the best of our knowledge, our finding is the second report of this compound from this genus. Compound 4 was isolated from B. ferruginea [15]. Thus, we can say that these compounds indicated a close phylogenetic relationship among Bridelia species. Compound 2 was possibly a newly characterized compound from the Bridelia genus as well as from the family Phyllanthaceae, which provides the prospect for further chemotaxonomic studies.
Experimental
General Experimental Procedure. The solvent of the mixture of plant powder soaked in methanol was evaporated using a Buchii Rotavapor rotary evaporator at 40° temperature and low pressure. For vacuum liquid chromatography (VLC), fine VLC grade silica (Kieselgel 60H) up to 6 cm was used for packing the column. The column was washed with petroleum ether (PE) to ensure that the packing column was compact. Gel permeation chromatography (GPC) technique was used, mainly to separate constituent compounds in a mixture, according to their molecular size as well as their relative polarity. The column was packed with Sephadex LH-20, which was soaked in chloroform for 24 h prior to packing for proper swelling. Thin-layer chromatography (TLC) was performed using aluminum sheets precoated with 0.20 mm silica gel 60 with fluorescent indicator UV254 (Macherey–Nagel, Germany). Spots on the TLC plates were visualized by examining under short and long-wave UV light and spraying with a vanillin-sulfuric acid solution followed by heating. Preparative thin-layer chromatography (PTLC) was used to purify the final compound. Glass TLC plates were prepared by coating with silica gel (Kieselgel 60 F254) in the laboratory for PTLC. NMR spectra were measured on a Bruker Avance 400 MHz machine for PMR and 2D NMR such as DEPT-135, COSY, HSQC, HMBC, etc., and 100 MHz for 13C NMR in CDCl3 for structure elucidation. The chemical shifts (δ) and coupling constants (J) for 1H and 13C NMR were recorded in ppm and Hz, respectively. HR-ESI mass analyzer was used to determine the mass of the molecular ion as well as fragmented ions.
Collection and Identification of Plant Material. The leaves of Bridelia stipularis (L.) Blume were collected in October 2017 from the Gazipur district of Bangladesh. The plant was identified by an expert from Bangladesh National Herbarium (BNH), and a voucher specimen was deposited (DACB accession number 45394) there.
Extraction and Fractionation of the Extract. After proper cleaning from dirt, the leaves were subjected to shade drying for a week. The dried leaves were cut into small pieces and then crushed into a coarse powder by a high-capacity grinding machine with proper care. About 1400 g of powdered plant material was taken in an amber-colored bottle and soaked with distilled methanol for 15 days with occasional shaking and stirring. The mixture was then filtered using a fresh cotton plug. After evaporation of methanol, dichloromethane (DCM) was added to it, and the whole mixed well. Then the DCM-soluble part was separated by using a Pasteur pipette, resulting in the separation of the non-polar DCM fraction. The amount of dried DCM fraction was 38.9 g, which was subjected to vacuum liquid chromatography (VLC) for further separation.
Isolation of Compounds. The 80% dichloromethane (DCM) in the petroleum ether (PE) fraction of VLC (Frs. 13–14) was subjected to CC with Sephadex LH-20 with a mixed solvent system of petroleum ether in chloroform (20%, 10%, and 5%, v/v) followed by methanol in chloroform (1%, 2%, 5%, 10%, 20%, and 50%, v/v). The eluents were analyzed by TLC, and Subfrs. 78–81 (eluted at 1% MeOH in CHCl3) were subjected to PTLC (70% ethyl acetate in toluene) to yield compound 1 (7.9 mg). Repeated washing of white crystals from Subfrs. 66–69 gave compound 2. PTLC (40% ethyl acetate in toluene) of Subfrs. 49–51 and 41–43 yielded compound 3 as a white amorphous powder and 4 as a yellowish solid, respectively. The 90% DCM in the PE fraction of VLC (Fr. 15) was subjected to CC with Sephadex LH-20, followed by PTLC of Subfrs. 55–58, to yield compound 5 as a yellowish solid.
Stipularone (1). Colorless liquid. HR-ESI-MS m/z: 155.0697 [M + H]+, 153.0559 [M – H]– (calcd for C8H10O3, 154.16). For 13C, 1H NMR, COSY, and HMBC spectral data, see Table 1.
3-(4′-Hydroxyphenyl)-2-propenoic Acid (4′′-Carboxyl)-phenyl Ester (2). White crystals. 1H NMR (400 MHz, CDCl3, δ, ppm, J/Hz): 6.22 (1H, d, J = 15.6, H-2), 6.51 (s, 4′-OH), 6.81 (2H, d, J = 8.4, H-3′, 5′), 6.92 (2H, d, J = 8.8, H-2′′, 6′′), 7.39 (2H, d, J = 8.4, H-2′, 6′), 7.60 (1H, d, J = 16.0, H-3), 7.76 (2H, d, J = 8.4, H-3′′, 5′′).
β -Amyrin (3). White amorphous powder. 1H NMR (400 MHz, CDCl3, δ, ppm, J/Hz): 0.78 (1H, d, J = 10.0, H-5), 0.80 (3H, s, H-25), 0.82 (3H, s, H-23), 0.88 (3H, s, H-30), 0.95 (3H, s, H-29), 1.01 (3H, s, H-24), 1.11 (3H, s, H-26), 1.16 (3H, s, H-28), 1.28 (3H, s, H-27), 1.92 (2H, m, H-22), 1.94 (2H, dd, J = 3.6, 9.0, H-16), 2.06 (2H, dd, J = 4.3, 13.2, H-19), 3.24 (1H, dd, J = 4.8, 10.8, H-3), 5.28 (1H, t, J = 3.4, H-12).
Scopoletin (4). Yellowish solid. 1H NMR (400 MHz, CDCl3, δ, ppm, J/Hz): 3.98 (3H, s, OMe), 5.72 (s, 7-OH), 6.29 (1H, d, J = 9.6, H-3), 6.87 (1H, s, H-8), 6.95 (1H, s, H-5), 7.62 (1H, d, J = 9.2, H-4).
4-Hydroxybenzoic Acid (5). White crystals. 1H NMR (400 MHz, CDCl3, δ, ppm, J/Hz): 6.49 (s, 4-OH), 6.89 (2H, d, J = 8.8, H-3, 5), 7.73 (2H, d, J = 8.8, H-2, 6).
References
P. Hoffmann, H. Kathriarachchi, and K. J. Wurdack, Kew Bull., 61, 37 (2006).
R. Samuel, H. Kathriarachchi, P. Hoffmann, M. H. J. Barfuss, K. J. Wurdack, C. C. Davis, and M. W. Chase, Am. J. Bot., 92, 132 (2005).
K. J. Wurdack, P. Hoffmann, R. Samuel, A. D. Bruijn, M. V. D. Bank, and M. W. Chase, Am. J. Bot., 91, 1882 (2004).
T. A. Ngueyem, G. Brusotti, G. Caccialanza, and P. V. Finzi, J. Ethnopharmacol., 124, 339 (2009).
A. Anjum, M. Z. Sultan, M. A. A. Sikder, C. M. Hasan, M. A. Al-Mansoor, and M. A. Rashid, Dhaka Univ. J. Pharm. Sci., 15, 221 (2016).
T. Khisha, R. Karim, S. R. Chowdhury, and R. Banoo, Bangladesh Pharm. J., 15, 59 (2012).
A. S. Sankaranarayanan, Anc. Sci. Life, 7, 175 (1988).
V. Gomathi and B. Jaykar, J. Pharm. Res., 9, 190 (2015).
A. Raymakers and F. Compernolle, Phytochemistry, 12, 2287 (1973).
F. Niu, Z. Cui, H. T. Chang, Y. Jiang, F. K. Chen, and P. F. Tu, Chin. J. Chem., 24, 1788 (2006).
M. O. Dias, L. Hamerski, and A. C. Pinto, Quim. Nova, 34, 704 (2011).
A. El-Demerdash, A. M. Dawidar, E. M. Keshk, and M. Abdel-Mogib, Rev. Latinoam. Quím., 37, 65 (2009).
J. Y. Cho, J. H. Moon, K. Y. Seong, and K. H. Park, Hull. Biosci. Biotechnol. Biochem., 62, 2273 (1998).
A. Deng and H. Qin, Chin. J. Chin. Mater. Med., 33, 158 (2008).
A. C. Ezike, P. A. Akah, E. M. Nnamani, C. O. Okoli, F. U. Ojike, F. S. Eze, C. E. Chime, and I. J. Azosiri, J. Complement Integr. Med. 8, 1 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2021, pp. 389–391.
Rights and permissions
About this article
Cite this article
Shahriar, K.R., Hossain, M.E., Rahman, K.M. et al. A New Cyclohexanone Derivative from the Leaves of Bridelia stipularis. Chem Nat Compd 57, 455–458 (2021). https://doi.org/10.1007/s10600-021-03386-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-021-03386-y