A new polyketide, 9(E)-3-(5,8-dihydroxynon-3-en-1-yl)-5,6-dihydroxy-2,6-dimethylcyclohex-2-enone (1), and one known compound, 5-chloroisorotiorin (2), were isolated from the mangrove-derived fungus Phomopsis sp. TJM1-5. Their structures were determined by extensive NMR and MS analyses, while the absolute configurations of C-5 and C-6 were established based on experimental and calculated electronic circular dichroism spectra. Compound 2 was isolated from the genus of Phomopsis for the first time. Compounds 1 and 2 were evaluated for their antibacterial activity against five pathogenic bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Marine-derived fungi have proved to be a rich source of new natural products, and the secondary metabolites from marine-derived fungi usually have novel structures and potent bioactivities, which have attracted the attention of chemists and pharmacologists [1,2,3]. In particular, the genus Phomopsis can produce various secondary metabolites with structural diversities and interesting bioactivities, such as cytotoxic phomeroids A and B [4], anti-inflammatory libertellenone T [5], antibacterial 5-methoxy-2-methyl-7-(3-methyl-2-oxobut-3-enyl)-1-naphthaldehyde [6], antifungal cyclo(D-Pro-L-Tyr-L-Pro-L-Tyr) [7], and phomopsichins A–D that inhibit α-glucosidase [8]. On the basis of the above results and in order to find new bioactive secondary metabolites from mangrove-derived fungus [3, 9, 10], the chemical constituents of the fungus Phomopsis sp. TJM1-5 were studied. As a result, a new polyketide (1) and one known polyketide (2) were isolated. Compound 2 was isolated from the genus Phomopsis for the first time. Herein we describe the isolation, structural characterization, and antibacterial activity of both compounds.

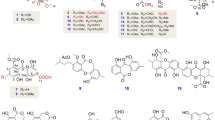
Compound 1 was obtained as a white amorphous powder. Its molecular formula was determined as C17H28O5 by HR-ESI-MS, requiring four degrees of unsaturation. The 1H NMR data (Table 1) showed resonances for two olefinic protons at δ 5.51 (m, H-9) and 5.50 (m, H-10), three oxygenated methine groups at δ 3.85 (dd, J = 10.0, 5.6 Hz, H-5), 3.83 (m, H-11), and 3.73 (m, H-14), three methyl signals at δ 1.79 (s, H-16), 1.19 (s, H-17), and 1.14 (d, J = 6.2 Hz, H-15), along with five methylene groups at δH 2.79 (dd, J = 16.8, 5.6 Hz, H-4β), 2.48 (overlapped, H-4α), 2.45 (m, H-7), 2.15 (m, H-8), 2.14 (m, H-13), and 1.57 (m, H-12).
The 13C NMR and DEPT data (Table 1) of 1 revealed the presence of 17 carbon signals, including one carbonyl carbon (δ 203.5), two double bonds (δ 155.6, 133.3, 130.8, and 128.3), three oxygenated methine carbon groups (δ 73.2, 70.4, and 68.6), one oxygenated quaternary carbon (δ 78.3), five methylene carbon groups (δ 43.7, 43.4, 39.1, 38.9, and 29.9), and three methyl carbons (δ 22.9, 17.9, and 11.8). The 13C NMR data (Table 1) at 203.5 (C), 130.8 (C), 155.6 (C) indicated the presence of an α,β-unsaturated ketone [11]. The 1H–1H COSY correlations revealed three coupling systems, CH2(4)-CH(5), CH2(7)-CH2(8)-CH(9)-CH(10), and CH(11)-CH2(12)-CH2(13)-CH(14)-CH3(15) (Fig. 1). The 1H–1H COSY correlations as well as the HMBC correlations of H-4 to C-2/6, H-7 to C-2/4, 16-Me to C-1/2/3, and 17-Me to C-1/5/6 confirmed the planar structure of 1. In the NOESY spectrum, the NOE correlations of Me-17/H-4α combined with the relatively large coupling constants between H-5 and H2-4 (JH-5,4α = 10.0 Hz, JH-5,4β = 5.6 Hz) indicated 5-OH and 6-OH to be α- and β-oriented, respectively [12]. The NOE correlations of H-8/H-10 and H-9/H-11 indicated the E configuration of the double bond between C-9 and C-10. The absolute configurations of C-5 and C-6 in 1 were established by the experimental and quantum chemical calculated electronic circular dichroism (ECD) spectra (Fig. 2). The ECD was calculated using TDDFT at the B3LYP/6-311+G(d,p) level with the CPCM model in MeOH. As shown in Fig. 2, the good agreement between the experimental and calculated ECD spectra of the (5S,6R)-1 led to the absolute configuration assignment. We used the modified Mosher’s method to determine the absolute configurations of C-11 and C-14 in 1, with the two carbons (C-11 and C-14) closed together, but we found that it was not accurate to calculate the 1H NMR chemical shifts between the (S)- and (R)-MTPA esters to determine the absolute configurations of C-11 and C-14 in 1. Thus, the structure of 1 was determined and named 9(E)-3-(5,8-dihydroxynon-3-en-1-yl)-5,6-dihydroxy-2,6-dimethylcyclohex-2-enone.
The isolated known compound 2 was identified as 5-chloroisorotiorin [13] by comparing the 1H, 13C NMR, and MS spectra data and the optical rotation data with the literature.
Compounds 1 and 2 were tested for their antibacterial activities against five pathogenic bacteria, Staphylococcus aureus, S. albus, Bacillus cereus, Escherichia coli, and Vibrio parahaemolyticus, but no activity was observed at the concentration of 50 μg/mL.
Experimental
General Experimental Procedures. Optical rotations were measured on a JASCO P-1020 digital polarimeter. IR spectra were recorded on a Thermo Nicolet 6700 (using KBr disks) spectrophotometer (Thermo Scientific, Madison, WI, USA). 1D and 2D NMR spectra were measured on a Bruker AV-400 (Bruker Corporation, Switzerland) instrument with tetramethylsilane as the internal standard. HR-ESI-MS spectra were obtained on a Bruker Daltonics Apex-Ultra 7.0 T (Bruker Corporation, Billerica, MA, USA) and a Q-TOF Ultima Global GAA076 LC mass spectrometer. A Waters 1525 prep-HPLC system with a Waters C18 semipreparative column (9.4 × 250 mm, 7 μm) was used for preparative HPLC. Sephadex LH-20 (Pharmacia Co., Ltd., Sandwich, UK) and silica gel (200–300 and 300–400 mesh, Qingdao Marine Chemical Factory, Qingdao, China) were used for column chromatography (CC). All solvents used were of analytical grade (Guangzhou, China).
Fungal Material. The fungal strain Phomopsis sp. TJM1-5 was isolated from the mangrove Acanthus ilicifolius collected from the South China Sea in August 2017. It was deposited in the Key Laboratory of Tropical Medicinal Resource Chemistry of Ministry of Education, People’s Republic of China. The strain was identified based on the analysis of the ITS sequence. The sequence data have been submitted to GenBank with the accession number MT071116. The fungal strain was cultivated in 8 L of potato liquid medium (33.3 g of sea salt in 1 L of potato infusion in 1 L Erlenmeyer flasks each containing 300 mL of culture broth) at 25°C without shaking for 4 weeks.
Extraction and Isolation. The fungal cultures were filtered through cheesecloth, and the filtrate was extracted with EtOAc (3 × 8 L, 24 h each). The organic extracts were concentrated in vacuo to yield an oily residue (1.7 g), which was subjected to silica gel column chromatography (petroleum ether, EtOAc, gradient 100:0–0:100) to give six fractions (Frs. 1–6). Fraction 3 (1.5 g) was subjected to silica gel column chromatography (200–300 mesh), Sephadex LH-20 column chromatography (petroleum ether–CHCl3–MeOH, 2:1:1), and semipreparative HPLC (MeOH–H2O, 80:20, 2.0 mL/min) to give compound 2 (5.9 mg). Fraction 4 (1.6 g) was subjected to repeated silica gel column chromatography (200–300 mesh, 300–400 mesh) and semipreparative HPLC (MeOH–H2O, 75:25, 2.0 mL/min) to afford compound 1 (2.6 mg).
9( E )-3-(5,8-Dihydroxynon-3-en-1-yl)-5,6-dihydroxy-2,6-dimethylcyclohex-2-enone (1). White amorphous powder; \( {\left[\alpha \right]}_{\mathrm{D}}^{24} \) –21.0° (c 0.15, MeOH). UV (MeOH, λmax, nm) (log ε): 200 (2.15), 219 (2.00). IR (KBr, νmax, cm–1): 3242, 1716, 1627, 1523, 1462, 1285, 886, 832, 667. HR-ESI-MS m/z 335.1819 [M + Na]+ (calcd for C17H28O5Na, 335.1824). For 1H and 13C NMR data (CD3OD), see Table 1.
Antibacterial Activity. All compounds were tested for their antibacterial activity. Antibacterial activity was determined against five pathogenic bacteria, Staphylococcus aureus (ATCC 25923), S. albus (ATCC 8032), Bacillus cereus (ATCC 14579), Escherichia coli (ATCC 35218), and Vibrio parahaemolyticus (ATCC17749), by the microplate assay method [14]. Ciprofloxacin was used as positive control.
References
R. Carroll, B. R. Copp, R. A. Davis, R. A Keyzer, and M. R. Prinsep, Nat. Prod. Rep., 36, 122 (2019).
L. Liu, Y. Y. Zheng, C. L. Shao, and C. Y. Wang, Mar. Life Sci. Technol., 1, 60 (2019).
C. J. Zheng, M. Bai, X. M. Zhou, G. L. Huang, T. M. Shao, Y. P. Luo, Z. G. Niu, Y. Y. Niu, G. Y. Chen, and C. R. Han, J. Nat. Prod., 81, 1045 (2018).
S. D. Chen, Z. M. Liu, H. B. Tan, Y. C. Chen, S. N. Li, H. H. Li, S. Zhu, H. X. Liu, and W. M. Zhang, Org. Chem. Front., 7, 557 (2020).
K. Xu, X. A. Zhang, J. W. Chen, Y. Shen, N. Jiang, R. Tan, R. Xiang, R. H. Jiao, and H. M. Ge, Tetrahedron Lett., 60, 15145 (2019).
X. M. Li, Y. C. Zeng, J. H. Chen, Y. K. Yang, J. Li, L. Ye, G. Du, M. Zhou, Q. F. Hu, G. Y. Yang, H.Y. Yang, and Y. Q. Duan, Chem. Nat. Compd., 55, 618 (2019).
S. Huang, W. J. Ding, C. Y. Li, and D. G. Cox, Pharmacogn. Mag., 10, 410 (2014).
M. X. Huang, J. Li, L. Liu, S. Yin, J. Wang, and Y. Lin, Mar. Drugs, 14, 215 (2016).
L. J. Yang, H. X. Liao, M. Bai, G. L. Huang, Y. P. Luo, Y. Y. Niu, C. J. Zheng, and C. Y. Wang, Nat. Prod. Res., 32, 208 (2018).
H. X. Liao, C. J. Zheng, G. L. Huang, R. Q. Mei, X. H. Nong, T. M. Shao, G. Y. Chen, and C. Y. Wang, J. Nat. Prod., 82, 2211 (2019).
Z. J. Lin, T. J. Zhu, Y. C. Fang, Q. C. Gu, and W. M. Zhu, Phytochemistry, 69, 1273 (2008).
Y. Ren, L. H. Chao, J. Sun, X. N. Chen, H. N. Yao, Z. X. Zhu, D. Dong, T. Liu, P. F. Tu, and J. Li, Nat. Prod. Res., 33, 347 (2019).
L. Pairet, S. K. Wrigley, I. Chetland, E. E. Reynolds, M. A. Hayes, J. Holloway, A. M. Ainsworth, W. Katzer, X. M. Cheng, D. J. Hupe, P. Charlton, and A. M. Doherty, J. Antibiot., 48, 913 (1995).
C. G. Pierce, P. Uppuluri, A. R. Teistan, J. F. L. Wormley, E. Mowa, G. Ramage, and J. L Lopez-ribot, Nat. Protoc., 3, 1494 (2008).
Acknowledgment
This work was supported by the Key Research and Development Program of Hainan Province (No. ZDYF2018183), National Natural Science Foundation of Hainan Province (No. 218MS045), the National Natural Science Foundation of China (Nos. 31760093 and 81660584), Guangxi Natural Science Foundation (No. 2016GXNSFBA380011), Program for Innovative Research Team in University (No. IRT-16R19), and Innovation Project of Postgraduate (No. Hys2018-75).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2021, pp. 50–52.
Rights and permissions
About this article
Cite this article
Zhu, XC., Chen, SJ., Huang, GL. et al. A New Polyketide from the Mangrove-Derived Fungus Phomopsis sp. TJM1-5. Chem Nat Compd 57, 59–62 (2021). https://doi.org/10.1007/s10600-021-03282-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-021-03282-5