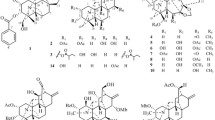
A new indole alkaloid (1), together with eight known indole alkaloid derivatives (2–9), was isolated from Hosta plantaginea for the first time. The structures of compounds 1–9 were elucidated on the basis of comprehensive spectroscopic analyses and references. Compounds 1–9 were evaluated for their inhibition of steroid 5α-reductase activity in vitro. Among them, compounds 1 and 2 showed important inhibition of steroid 5α-reductase activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hosta plantaginea is an important Mongolian medicine among the Chinese population, and it belongs to the Liliaceae family [1]. H. plantaginea has been used as a traditional Chinese medicine for the treatment of several illnesses, including sore throat, pulmonary fever, hoarseness, and toxic fever in clinic [2]. Modern pharmacological studies have shown that the extracts or individual components of H. plantaginea exhibited anti-inflammatory [3], antitumor [4], antioxidant [5], antibacterial [6], and hepatoprotective [7] activities. Previous phytochemical investigations suggested that H. plantaginea contained alkaloids [8], favonoids [9], steroids [10], and other compounds. As part of our further investigation of its bioactive constituents, H. plantaginea was exhaustively studied for its inhibition of steroid 5α-reductase activity performed as a bioassay-guided investigation. As a result, a new indole alkaloid (1), together with eight known indole alkaloid derivatives (2–9), was isolated and identified from their spectral data and comparison with references for the first time (Fig. 1).
Compound 1 was obtained as a colorless powder, and its molecular formula was deduced as C22H18N2O5 by HR-ESI-MS at m/z 391.1227 [M + H]+ (calcd for C22H19N2O5, 391.1294), corresponding to 15 degrees of unsaturation. The UV spectrum of compound 1 showed absorptions at λmax 210, 235, 288, and 335 nm, revealing the existence of the β-carboline chromophore [11]. The IR spectrum exhibited absorptions of hydroxyl (3352 cm–1), aromatic ring (1628 cm–1), and methyl (1354 cm–1) functionalities.
The 1H NMR spectrum of compound 1 (Table 1) showed a typical ABX system at δ 8.11 (1H, d, J = 1.6 Hz, H-6), 7.03 (1H, dd, J = 8.4, 1.6 Hz, H-8), and 7.52 (1H, d, J = 8.4 Hz, H-9) (Table 1), and a typical singlet at δH 6.09 (2H, s, H-5) in the 1H NMR spectrum and δC 101.8 (C-5) in the 13C NMR spectrum, which revealed that the -O-CH2-O- fragment was in compound 1 according to the HSQC and HMBC spectra. Moreover, the -CH=CHCOCH3 fragment (δH 7.57, d, J = 15.9 Hz, H-1′′; 6.70, d, J = 15.9 Hz, H-2′′; 2.35, s, H-4′′; δC 197.8, C-3′′) [12] was assigned to C-7 by the correlations of H-6 to C-1′′/4a, H-1′′ to C-8, and H-2′′ to C-7 in the HMBC spectrum (Fig. 1). A typical furan ring (δH 7.07, d, J = 3.5 Hz, H-3′; 6.61, d, J = 3.5 Hz, H-4′) [13] was connected to C-1 by the correlation of H-3′ to C-1 in the HMBC spectrum (Fig. 1). The other fragments of compound 1 were determined by the HMBC correlations of H-5 to C-3/4, H-6 to C-4a, H-8 to C-6, H-9 to C-7, and H-4' to C-2′/6′ (Fig. 1) and the 1H–1H COSY correlations of H-8 to H-9, H-3′/H-4′, and H-1′′/H-2′′ (Fig. 1). Therefore, compound 1 was identified as (E)-1-[5′-(hydroxymethyl)furan-6′-yl)]-11-methyl-[1,3]dioxolo-pyrido-indole-but-2′′-en-3′′-one.
Additionally, eight known compounds (2–9) were identified by comparison of their spectroscopic data with references. They were determined as perlolyrine (2) [13], 1-[(3-acetoxymethyl)-2-furyl]-1,2,3,4-tetrahydro-9H-pyrido-[3,4-b] indole (3) [14], 2-(1-(furan-2-yl)-9H-pyrido[3,4-b]indol-3-yl)propan-2-ol (4) [15], taberniacin B (5) [16], taberniacin A (6) [16], harmine (7) [13], galanthindole (8) [17], and tryptamine (9) [14]. Meanwhile, the known isolates (2–9) were identified as indole alkaloid derivatives and isolated from H. plantaginea for the first time.
Meanwhile, compounds 1 and 2 showed significant inhibition of steroid 5α-reductase activities in vitro. In this paper, we report the isolation and characterization of compounds 1–9 and their inhibition of steroid 5α-reductase activities in vitro.
Experimental
General Procedures. The UV and IR spectral data were measured by an Australia GBC UV-916 spectrophotometer and a Nicolet 5700 FT-IR spectrometer with KBr pellets, separately. The NMR spectra were performed on Bruker-400 and Bruker Avance III HD-600 spectrometers with TMS as internal standard at 25°C. The HR-ESI-MS data were recorded on an Agilent 1100 series LC/MSD ion trap mass spectrometer, and the ESI-MS data were measured by an LTQ Orbitrap XL spectrometer. The HPLC separation was performed on an Agilent 1200 series spectrometer with a DIKMA (4.6 . 250 mm) analytical column packed with C18 (5 μm), and the semipreparative HPLC was conducted on a CXTH-LC3050N instrument with a DAD detector and an YMC-Pack ODS-A column (250 . 10 mm, S-5 μm, 12 nm). The column chromatography was performed on silica gel (100–200, 200–300 mesh), Sephadex LH-20, Toyopearl HW-40C, and RP-C18 silica gel (60 mm) columns. The TLC experiments were conducted on GF254 silica gel plates, and the spots were visualized under UV light (254 or 365 nm) or by spraying with 10% H2SO4 in 95% EtOH followed by heating [18].
Plant Material. H. plantaginea was purchased from Anguo City, Hebei Province, China, in March 2019. The plant was identified and authenticated by Prof. Guoyue Zhong of Jiangxi University of Traditional Chinese Medicine. A voucher specimen (No. YZH-201903) has been deposited in Jiangxi University of Traditional Chinese Medicine, Nanchang 330004, China.
Extraction and Isolation. The air-dried flowers of H. plantaginea (13.5 kg) were extracted with 95% EtOH (30 L) and 70% EtOH (30 L) under reflux three times. The obtained filtrates were concentrated by a rotary evaporator under reduced pressure to obtain a black residue (1.3 kg). The extract was dissolved in water and partitioned with petroleum ether, EtOAc, and n-BuOH, separately, yielding the petroleum ether layer (243.8 g), the EtOAc layer (207.2 g), and the n-BuOH layer (312.5 g). It was found that the EtOAc soluble fraction exhibited significant inhibition of steroid 5α-reductase activity with Ki =153.4 ± 5.7 nmol/L according to bioactivity-guided investigations. Consequently, the EtOAc soluble fraction was purified on a silica gel (100–200 mesh) column and eluted with petroleum ether–EtOAc (20:1→10:1→5:1→1:1) to obtain four fractions: A (30.7 g), B (52.4 g), C (67.2 g), and D (42.6 g), respectively. Among them, Fr. B exhibited inhibition of steroid 5α-reductase activity with Ki = 84.6 ± 2.9 nmol/L according to the results of pharmacological screening. Therefore, Fr. B was further purified on the silica gel (200–300 mesh) column with gradient elution (petroleum ether–EtOAc, 15:1→10:1→5:1) to afford three subfractions: B-1 (9.55 g), B-2 (17.65 g), and B-3 (11.52 g). Subfraction B-2 was fractionated on silica gel (100–200, 200–300 mesh), Sephadex LH-20 (CH2Cl2–MeOH, 1:1), and prep-HPLC, consecutively, yielding 5 (13.50 mg), 6 (9.56 mg), 8 (16.01 mg), and 9 (11.20 mg). In the same way, Subfr. B-3 was separated over silica gel (100–200, 200–300 mesh), Toyopearl HW-40C (95%MeOH), and prep-HPLC, repeatedly, yielding 1 (6.21 mg), 2 (9.55 mg), 3 (14.52 mg), 4 (8.04 mg), and 7 (12.72 mg).
Inhibition of Steroid 5α-Reductase Assay. In this experiment, the isotope screening method was used to determine the ability of compounds 1–9 to inhibit steroid 5α-reductase in vitro with epristeride as the positive control. The reaction solution (50 μL) consisted of NADPH (12.8 μmol/L), rat liver 5α-reductase microsomes (2 μL), Tris-HCl buffer, [3H] testosterone (0, 0.25, 0.50, 0.75, 0.10 μmol/L), and serial concentrations of epristeride or the tested compounds (0, 0.02, 0.04, 0.08, 0.10 μmol/L). After incubation for 20 min at 37°C, the reaction was terminated by addition of 10 μL ethyl acetate. [3H]Testosterone was identified by exposing the plate to iodine vapor. The fractions for each tube were scraped off and placed into counting vials along with 10 mL scintillation liquid. The radioactivity in each sample was measured by an LS 6500 multi-purpose scintillation counter. The 5α-reductase activity was expressed as DHT formed in 20 min, and the inhibition constant was calculated [19].
In this work, a new indole alkaloid (1), along with eight known isolates (2–9), was isolated from H. plantaginea for the first time, and the compounds (1–9) were identified as indole alkaloid derivatives by comprehensive spectroscopic analyses and comparison with references. Compounds 1–9 were evaluated for their ability to inhibit steroid 5α-reductase activity in vitro. The inhibition constants of the tested compounds are listed in Table 2. The compounds with Ki greater than 10000 nmol/L were considered to be ineffective. Among them, compounds 1 and 2 showed significant inhibition of steroid 5α-reductase activities with Ki = 32.4 ± 1.8 and 56.4 ± 5.6 nmol/L, respectively. However, compounds 3–9 have not exhibited inhibition of steroid 5α-reductase activities (Ki greater than 10000 nmol/L).
References
J. Feng, L. F. Hu, and Y. Tang, Pharm. Clin. Chin. Mater. Med., 59, 8 (2017).
J. W. He, L. Yang, and G. Y. Zhong, Chin. Trad. Herb. Drugs, 47, 4295 (2016).
J. Y. Qu, M. Y. Wang, C. M. Wang, G. Y. Zhong, and X. B. Li, Chin. Trad. Herb. Drugs, 42, 217 (2011).
J. Q. Liu, C. F. Wang, M. H. Qiu, and W. X. Hu, Chin. Trad. Herb. Drugs, 41, 520 (2010).
X. H. Bao, Q. H. Wang, B. Y. Q. E. Bao, J. J. Han, and W. L. J. Ao, Chem. Nat. Compd., 53, 614 (2017).
Y. Xin and Y. H. Bai, Chin. Trad. Pat. Med., 37, 653 (2015).
R. R. Wei, Q. G. Ma, G. Y. Zhong, J. W. He, and Z. P. Sang, J. Ethnopharmacol., 253, 112685 (2020).
Y. H. Wang, Z. K. Zhang, F. M. Yang, Q. Y. Sun, H. P. He, Y. T. Di, S. Z. Mu, Y. Lu, Y. Chang, Q. T. Zheng, M. Ding, J. H. Dong, and X. J. Hao, J. Nat. Prod., 70, 1458 (2007).
H. X. Xie, J. H. Zhang, H. G. Zhang, and P. F. Xue, Chin. Pharm. J., 44, 733 (2009).
J. H. Zhang, H. G. Xie, P. F. Xue, H. G. Zhang, X. Y. Liu, and M. Baijie, Chin. Pharm. J., 45, 335 (2010).
K. B. Wang, C. M. Yuan, C. M. Xue, D. H. Li, Y. K. Jing, H. P. He, X. J. Hao, Y. T. Di, Z. L. Li, and H. M. Hua, RSC Adv., 4, 53725 (2014).
C. Y. Wang, J. D. Hao, X. Y. Ning, J. S. Wu, D. L. Zhao, C. J. Kong, C. L. Shao, and C. Y. Wang, RSC Adv., 8, 4348 (2018).
C. Wu, L. J. He, X. Yi, J. Qin, Y. L. Li, Y. B. Zhang, and G. C. Wang, J. Nat. Med., 73, 667 (2019).
J. B. Bremner, W. Sengpracha, I. Southwell, C. Bourke, B. W. Skelton, and A. H. White, Aust. J. Chem., 57, 273 (2004).
G. Abbiati, E. M. Beccalli, A. Marchesini, and E. Rossi, Synthesis, 16, 2477 (2001).
Y. Hirasawa, X. Dai, J. Deguchi, S. Hatano, T. Sasaki, R. Ohtsuka, A. E. Nugroho, T. Kaneda, and H. Morita, J. Nat. Med., 73, 627 (2019).
M. Safratova, A. Hostalkova, D. Hulcova, K. Breiterova, V. Hrabcova, M. Machado, D. Fontinha, M. Prudencio, J. Kunes, J. Chlebek, D. Jun, M. Hrabinova, L. Novakova, R. Havelek, M. Seifrtova, L. Opletal, and L. Cahlikova, Arch. Pharm. Res., 41, 208 (2018).
Q. G. Ma, R. R. Wei, M. Yang, X. Y. Huang, G. Y. Zhong, Z. P. Sang, J. H. Dong, J. C. Shu, J. Q. Liu, R. Zhang, J. B. Yang, A. G. Wang, T. F. Ji, and Y. L. Su, Bioorg. Chem., 86, 159 (2019).
J. Xin, W. S. Tian, Y. Y. Sun, and Z. H. Tu, Chin. J. Pharmacol. Toxicol., 17, 395 (2003).
Acknowledgment
This research program is financially supported by the National Natural Science Foundation of China (81803843), the Scientific Research Project of Jiangxi Administration of Traditional Chinese Medicine (2019A002, 2019A018), the Standard Revision Research Project of National Pharmacopoeia Committee (2018Z090), the Science and Technology Project of Jiangxi Provincial Department of Education (GJJ180688, GJJ180662), the Science and Technology Project of Jiangxi Health Commission (20195650, 20195648), the Scientific Research Project of First-class Discipline of Chinese Materia Medica of Jiangxi University of TCM (JXSYLXK-ZHYAO027, JXSYLXK-ZHYAO032), and the Doctoral Research Initiation Fund Project of Jiangxi University of TCM (2018BSZR010, 2018BSZR007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2020, pp. 760–762.
Rights and permissions
About this article
Cite this article
Wei, R., Ma, Q. Indole Alkaloids from Hosta plantaginea and Inhibition of Steroid 5α-Reductase Activities In Vitro. Chem Nat Compd 56, 888–891 (2020). https://doi.org/10.1007/s10600-020-03176-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-020-03176-y