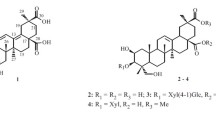
A new sesquiterpene lactone, namely, eupachiilide A (1), as well as two known sesquiterpene lactones, eupachinilide B (2), and eupalinilide G (3), were isolated from the whole plant of Eupatorium chinense L. The new structure was elucidated by spectral methods, especially 2D NMR techniques. The three natural compounds were all Michael addition acceptors, whose electrophilic moiety could react with the nucleophilic residues of the relevant active site and produce a variety of bioactivities. Eupachiilide A demonstrated potent cytotoxicity against two TNBC cell lines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Triple-negative breast cancer (TNBC) is a subgroup of breast cancer defined as estrogen receptor (ER)/progesterone receptor (PR)/human epidermal growth factor 2 (HER2) expression deficiency and accounts for approximately 15–20% of all breast cancers. Owing to lack of targeted therapies, TNBC exhibits an aggressive phenotype and lower survival rate in clinic, especially in female patients [1, 2]. Thus an innovative treatment is still needed for this nebulous disease.
The Michael addition acceptors (MAA) are well known as an electrophilic small molecule that can develop covalent modification of nucleophilic targets through their Michael acceptor properties ranging from transcription factors to various enzymes and receptors [3]. For example, a specific MAA-typed compound enone 6 selectively inhibits STAT3 phosphorylation without affecting STAT1 phosphorylation and cytostatic effect in human breast epithelial cells, exhibiting cancer cell-specific inhibitory properties [4]. Structurally, natural MAA-typed sesquiterpene lactones commonly consist of a sesquiterpene configuration and a quinary unsaturated lactone ring, thus rendering the molecules highly active to nucleophilic attacks and exerting the bioactivities as the Michael receptor moiety. There have been reports that MAA-typed sesquiterpene lactones contained in Eupatorium L. can effectively inhibit TNBC cells in vitro [5, 6].
The genus Eupatorium L. (Compositae) attracts considerable attention due to its rich and valuable source of bioactive flavonoids and terpenoids [7, 8], with the latter comprising mainly sesquiterpenes [9]. Eupatorium chinense L. is typical of this genus, which has a bitter taste and is indigenous to China [10] and traditionally prescribed for the treatment of cold, snakebite, and inflammation by local residents [11]. MAA-typed sesquiterpene lactones are considered to be a class of compounds rich in the genus Eupatorium L. with a broad range of significant pharmacological activities, such as anti-inflammatory, antibiotic, antitumor, antitrypanosomal, and cell cytotoxic effects [12,13,14,15,16]. Thus, the various impressive chemical entities from this genus are constantly exhibiting excellent drug prospects due to their abundant structures and interesting bioactivities.

We studied the ethyl acetate extract of E. chinense, and identified three MAA-typed sesquiterpene lactones, which are reported herein, including a new compound, eupachiilide A (1) and two known compounds, eupachinilide B (2) and eupalinilide G (3). The isolation and structural elucidation of the new compound and the anti-TNBC activities of 1–3 are described as well.
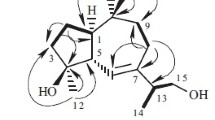
Eupachiilide A (1), colorless oil, \( {\left[\upalpha \right]}_{\mathrm{D}}^{25} \) –103.0° (c 0.10, MeOH). The molecular formula was determined to be C22H26O9 based on ESI-QTOF-MS with an [M + Na]+ ion at m/z 457.1475 (calcd 457.1467) and an [M + H]+ ion at m/z 435.1655 (calcd 435.1654), inferring 10 degrees of unsaturation. The 1H and 13C NMR together with DEPT spectra showed the presence of three methyls, four secondary carbons, eight tertiary carbons, and seven quaternary carbons. One wide IR absorption band at 3487 cm–1 and another set of strong peaks 1760 cm–1 and 1710 cm–1 collectively indicated that the compound contained hydroxyl and ester carbonyl groups. A set of proton signals at δ 6.37 (1H, d, J = 3.5 Hz, H-13) and 5.59 (1H, d, J = 3.0 Hz, H-13) and carbon signals at δ 133.5 (C-11) and 123.3 (C-13) was attributed to a exocyclic double bond between C-11 and C-13, combined with a carbonyl ester at δC 169.0 (C-12), indicating the presence of a five-membered unsaturated lactone ring (Table 1). One epoxy three-membered ring on the position of C-3 and C-4 was distinguished by characteristic signals of δH 3.41 (1H, s, H-3), δC 62.1 (C-3), and 66.1 (C-4), and the other epoxy three-membered ring on C-10, C-14 was identified through δH 2.71 (1H, d, J = 4.7 Hz, H-14), 2.68 (1H, d, J = 4.7 Hz, H-14), δC 54.5 (C-10), and 56.6 (C-14). Considering the 1H NMR and 13C NMR, combined with the literature, we concluded that the compound has the structural core of guaiane-type sesquiterpenoids [17, 18]. The structural core was consistent with the reported literature [19]. In addition, the substituent group with proton signals at δ 6.73 (1H, t, J = 5.9 Hz, H-3′), 4.36 (2H, d, J = 5.9 Hz, H-4′), and 1.79 (3H, s, H-5′) and carbon signals at δ 166.4 (C-1′, ester carbonyl), 127.7 and 141.3 (C-2′, 3′, double bond), 59.7 (C-4′), and 12.8 (C-5′) was assignable to a 4′-hydroxytigloyl group [20,21,22]. Furthermore, the substituent group signal at δC 166.4 and the proton signal at δ 5.61 (1H, ddd, J = 8,8, 7.4, 3.7 Hz, H-8) had HMBC remote correlation, suggesting that the 4′-hydroxytigloyl moiety was attached at C-8. In the HMBC (Fig. 1), the acetyl group at δH 2.12 (3H, s, H-2′′) and δC 169.5 (C-1′′), 21.3 (C-2′′) was linked to C-2 owing to the HMBC correlation between δH 5.39 (1H, d, J = 5.5 Hz, H-2) and δC 169.5.
The relative configuration of 1 was determined via NOESY experiments (Fig. 1). H-6 (δ 4.77, 1H, dd, J = 11.6, 8.2 Hz) was β-oriented and H-7 (δ 3.58, 1H, dd, J = 8.2, 3.7 Hz) was α-oriented since this is the typical stereoconfiguration in natural guaiane-type sesquiterpenoids [23]. Based on this characteristic, the NOESY correlation pairs of H-7/H-8, H-7/H-5, and H-7/H-1 suggested that H-8, H-5 (δ 2.60, 1H, dd, J = 11.6, 8.6 Hz) and H-1 (δ 1.96, 1H, dd, J = 8.6, 5.5 Hz) were all in the α-orientation.
In conclusion, the structure of 1 was elucidated as (E)-(2R,3a′S,4′R,6a′S,7′S,7a′R,8a′S,8b′S,8c′S)-7′-acetoxy-8a′-methyl-3′- methylene-2′-oxodecahydro-2′H-spiro[oxirane-2,6′-oxireno[2′,3′:2,3]azuleno[4,5-b]furan]-4′-yl-4-hydroxy-2-methylbut-2-enoate.
The structures of compounds 2–3 were determined by analysis of their NMR spectroscopic data and comparison with the literature data reported. They were identified as eupalinilide G (2) [24] and eupachinilide B (3) [10], respectively.
To determine the inhibitory effect of compounds 1–3 on two TNBC cell lines in vitro, an MTT assay was employed. The results showed that eupachiilide A demonstrated potent cytotoxicity on MDA-MB-231 and MDA-MB-468 cell lines. The IC50 of compounds 1–3 against TNBC cells are shown in Table 2.
The MTT result showed that eupachiilide A had potent cytotoxicity against two TNBC cell lines compared to adriamycin, followed by eupachinilide B, while eupalinilide G had poor activity against TNBC cell lines. Eupachiilide A is worthy of further study, and we will study the molecular mechanism of its anti-TNBC activity. Its anti-TNBC activity in vivo will also be studied by xenotransplantation of human tumor in nude mice. We will keep on studying the drug property of eupachiilide A.
EXPERIMENTAL
General Experimental Procedures. Optical rotation was measured on a Rudolph Autopol 1 automatic polarimeter (Rudolph, USA). IR spectrum was recorded on a Thermo Scientific Nicolet iS50 FT-IR spectrometer (Thermo, USA). Mass spectrometer analysis was performed on Waters SYNAPT G2-SI MS systems (Waters, USA) equipped with an ESI source and Q-TOF/MS. The 1H, 13C, and 2D NMR were performed with a Bruker Ultra Shield Plus 600 MHz spectrometer using TMS as internal standard. Medium-pressure liquid chromatography was carried out using a Buchi system on an ODS column (40–60 μm, Daiso Co., Ltd., Japan). Semipreparative HPLC was carried out using a Waters system consisting of a 600 pump and a 2487 detector, accompanied by an Amethyst C18 column (250 × 21.2 mm, 10 μm) purchased from Sepax Technologies (USA). Silica gel (200–300 mesh) was used for column chromatography (Qingdao Ocean Chemical Plant, Qingdao, China). Other chemicals and solvents were of analytical grade. The MDA-MB-231 and MDA-MB-468 cell lines were obtained from the Shanghai Institute of Materia Medical, Chinese Academy of Sciences (Shanghai, China). DMEM/F12 medium, trypsin, EDTA, and FBS were purchased from Gibco (Grand Island, NY, USA).
Plant Material. The whole plant of Eupatorium chinense L. was collected from Ruyijian of Xihu District, Hangzhou, China (E 120° 0′ 13′′, N 30° 7′ 4′′) and was identified by Prof. Ru-Song Zhang (Zhejiang Chinese Medical University, China).
A voucher specimen has been deposited with the Laboratory of Chemical Biology of Chinese Medicine in Zhejiang Chinese Medical University (No. ZY-EChL-20160915).
Extraction and Isolation. The dried whole plant of E. chinense (4.7 kg) was ground and percolated with 20-fold 95% EtOH at room temperature, then suspended in 1.5 L of warm water and partitioned with EtOAc (1.5 L × 3). After removal of the EtOAc under reduced pressure, a dark green residue (261 g) was obtained. The EtOAc of E. chinense (135 g) was subjected to silica gel (200–300 mesh) column chromatography and eluted with a gradient solvent system of petroleum ether–EtOAc (30:1–0:1) to give eight fractions (Frs. A–H). Fraction G (3.0 of 24.3 g) was separated by MPLC using MeOH/H2O as the mobile phase (40%, 45%, 55%, and 65%, each 600 mL, flow rate 30 mL/min). Subsequently, fraction 10 from the 45% MeOH (78.6 mg) fraction was separated by semipreparative HPLC (45% MeOH, flow rate 10 mL/min) to obtain compound 1 (19.4 mg), 2 (14.3 mg). Fraction H (2.1 of 9.2 g) was flash-chromatographed sequentially over a silica gel column (petroleum ether–EtOAc, 2:1–0:1) and subjected to semipreparative HPLC (30% ACN, flow rate 10 mL/min) to obtain compound 3 (171.8 mg).
Antiproliferative Activities Assay on Two TNBC Cell Lines. The antiproliferative activities on MDA-MB-231 and MDA-MB-468 of compounds 1–3 were detected using the MTT assay, with adriamycin as positive control. TNBC cells were inoculated into 96-well cell culture plate for 24 h, then treated with different concentrations of compounds 1–3 and adriamycin for another 72 h. The cells were incubated with MTT solution for 4 h and dissolved with 150 μL DMSO. The relative cell viability was measured by scanning with a cell Imaging Multi-mode Reader (BioTek, CA, USA) using a 570 nm filter. IC50 values were calculated by 82798-IC50 software.
References
S. Narrandes, S. Huang, L. Murphy, and W. Xu, BMC Cancer, 18, 22 (2018).
S. Hurvitz and M. Mead, Curr. Opin. Obstet. Gynecol., 28, 59 (2016).
I. V. Maucher, M. Ruhl, S. B. M. Kretschmer, B. Hofmann, B. Kuhn, J. Fettel, A. Vogel, K. T. Flugel, G. Manolikakes, N. Hellmuth, A. K. Hafner, V. Golghalyani, A. K. Ball, M. Piesche, C. Matrone, G. Geisslinger, M. J. Parnham, M. Karas, D. Steinhilber, J. Roos, and T. J. Maier, Biochem. Pharmacol., 125, 55 (2017).
K. Kim, S. J. Kim, Y. T. Han, S. J. Hong, H. An, D. J. Chang, T. Kim, B. Lim, J. Lee, Y. J. Surh, and Y. G. Suh, Bioorg. Med. Chem. Lett., 25, 5444 (2015).
X. Yu, Q. Zhang, L. Tian, Z. Guo, C. Liu, J. Chen, W. Ebrahim, Z. Liu, P. Proksch, and K. Zou, J. Nat. Prod., 81, 85 (2018).
J. Chun, R. J. Li, M. S. Cheng, and Y. S. Kim, Cancer Lett., 357, 393 (2015).
P. Y. Liu, D. Liu, and W. H. Li, Chem. Biodiv., 12, 1481 (2015).
H. Hendriks, Th. M. Malingre, and E. T. Elema, Pharm. Weekbl. Sci., 5, 281 (1983).
K. Ito, Y. Sakakibara, and M. Haruna, Phytochemistry, 21, 715 (1982).
S. P. Yang, J. Huo, Y. Wang, L. G. Lou, and J. M. Yue, J. Nat. Prod., 67, 638 (2004).
S. P. Yang, J. G. Cheng, J. Huo, H. L. Jiang, K. X. Chen, and J. M. Yue, Chin. J. Chem., 23, 1530 (2005).
F. Wang, H. H. Zhong, S. Q. Fang, Y. F. Zheng, C. Y. Li, G. P. Peng, and X. C. Shen, Planta Med., 84, 123 (2018).
M. G. Repetto and A. Boveris, Mini-Rev. Med. Chem., 10, 615 (2010).
Y. Ren, U. M. Acuna, F. Jimenez, R. Garcia, M. Mejia, H. Chai, J. C. Gallucci, N. R. Farnsworth, D. D. Soejarto, E. J. Carcache de Blanco, and A. D. Kinghorn, Tetrahedron, 68, 2671 (2012).
T. Itoh, M. Oyama, N. Takimoto, C. Kato, Y. Nozawa, Y. Akao, and M. Iinuma, Bioorg. Med. Chem., 17, 3189 (2009).
J. Tasqiah, H. Yoshie, Z. Stefanie, K. Marcel, H. Matthias, and A. Michael, Fitoterapia, 82, 955 (2011).
M. Chadwick, H. Trewin, F. Gawthrop, and C. Wagstaff, Int. J. Mol. Sci., 14, 12780 (2013).
M. Maas, A. Hensel, F. B. Costa, R. Brun, M. Kaiser, and T. J. Schmidt, Phytochemistry, 72, 635 (2011).
K. Ito, Y. Sakakibara, and M. Haruna, Chem. Lett., 8, 1473 (1979).
D. Youssef and A. W. Frahm, Planta Med., 60, 267 (1994).
Luis R. Hernandez, Cesar A. N. Catalan, Carlos M. Cerda-Garcia-Rojas, and P. Joseph-Nathan, Phytochemistry, 37, 1331 (1994).
F. Bohlmann, Nezhun Ates (Goren), J. Jakupovic, R. M. King, and H. Robinson, Phytochemistry, 23, 1180 (1984).
W. Herz, H. Grisebach, and G. W. Kirby (eds.), Progress in the Chemistry of Organic Natural Products, Vol. 38, Springer, Vienna, 1979, pp. 47–390.
J. Huo, S. P. Yang, J. Ding, and J. M. Yue, J. Nat. Prod., 67, 1470 (2004).
Acknowledgment
The work was financially supported by the National Natural Science Foundation of China (81773868), the Natural Science Foundation of Zhejiang Province (LY17H280004), the Zhejiang Province Chinese medicine scientific research fund project (2016ZA050), and the Hangzhou Major Science and Technology Project (20172016A01).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 4, July–August, 2020, pp. 565–568.
Rights and permissions
About this article
Cite this article
Jiang, Ql., Shou, Pt., Sun, Mj. et al. A New Sesquiterpene Lactone from Eupatorium chinense and its Anti-TNBC Activity. Chem Nat Compd 56, 651–655 (2020). https://doi.org/10.1007/s10600-020-03114-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-020-03114-y