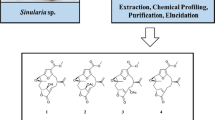
A new cembrane-type diterpenoid, sinulaflexiolide P (1), along with three known derivatives: sinulaflexiolide H (2), 11-epi-sinulariolide acetate (3), and (1S*,3S*,4S*,7E,11E)-3,4-epoxy-13-oxo-3,7,11,15-cembratriene (4), was isolated from a population of Bornean soft coral Sinularia flexibilis. The structures of these metabolites were elucidated based on spectroscopic data including NMR and HR-ESI-MS. In addition, these compounds were tested against six strains of marine fungi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Marine organisms are well known to be an essential source of bioactive natural products [1, 2]. Soft corals (Alcyoniidae) are recognized to be a rich source of sesquiterpenoids [3, 4], cembrane-based diterpenoids [5,6,7] including their dimers [8, 9], xenicane-type [10, 11] and to a lesser extent prenyleudesmane-derived diterpenoids [12, 13], eunicellin-based diterpenoids [14], cutibane-type diterpenoids [15], casbane-based diterpenoids [16], and prenylated germacrene-type diterpenoids [17], as well as meroditerpenoids [18], while terpenoids including sesquiterpenoids [19, 20], lobane-type diterpenoids [21, 22], cembranoids [19, 20, 23,24,25,26,27], and steroids [28] have been reported from soft coral belonging to the species Sinularia flexibilis. Some of these compounds exhibit cytotoxic [19, 20, 24, 25, 28], antibacterial [21, 23], anti-inflammatory [26, 27], and antifungal activities [20, 29]. Because of the great interest in this organism, the study of one population of Bornean soft coral Sinularia flexibilis collected from Mantanani Island (Sabah, Malaysia) has led to the isolation of one new cembrane-type diterpenoid, sinulaflexiolide P (1), along with three known derivatives, sinulaflexiolide H (2) [24], 11-epi-sinulariolide acetate (3) [30], and (1S*,3S*,4S*,7E,11E)-3,4-epoxy-13-oxo-3,7,11,15-cembratriene (4) [31]. This paper reports the isolation, structure elucidation, and antifungal potentials of these compounds.
Compound 1 was isolated as a colorless oil: \( {\left[\upalpha \right]}_{\mathrm{D}}^{25} \) –44.0° (c 0.20, CHCl3). Its molecular formula was determined as C21H32O5 based on HR-ESI-MS ions at m/z 387.2156 [M + Na]+ (calcd for C21H32O5Na, 387.2142), 365.2335 [M + H]+ (calcd for C21H33O5, 365.2323), and 347.2210 [M + H – H2O]+ (calcd for C21H31O4, 347.2217). The IR (KBr) absorption at 3400, 1720, 1650, and 1010 cm–1 indicated the presence of hydroxyl, carbonyl, and alkoxy groups in the molecule.

Upon careful comparison of NMR data (Table 1) between 1 and sinulaflexiolide G, we found a general similarity in their structure, except for the replacement of the ethoxy functionality (δC 60.6, 13.9; δH 4.10, 1.08) at C-16 in sinulaflexiolide G by a methoxy moiety (δC 52.1; δH 3.76) in 1 [24].
The planar structure was further confirmed by three consecutive 1H–1H spin systems, determined via an 1H–1H COSY experiment, which are connected through key HMBC cross peaks of H2-17 to C-1, C-15 and C-16; H3-18 to C-3, C-4 and C-5; H3-19 to C-7, C-8 and C-9; H3-20 to C-11, C-12, and C-13; and both H2-6 and H2-7 to C-5 (Fig. 1). The stereogenic centers at C-1, C-4, and C-13 were determined to be identical to those of sinulaflexiolide G upon examination of the chemical shifts [24].
Compounds 1–4 were screened against six fungal strains: Fusarium moniliforme (NJM 8995), F. oxysporum (NJM 0179), F. solani (NJM 8996), Haliphthoros milfordensis (IPMB 1603), H. sabahensis (IPMB 1402), and Lagenidium thermophilum (IPMB 1401). The MICs of compounds 1–4 were as follows: for F. moniliforme, F. oxysporum, and F. solani, > 50 μg/mL; for H. sabahensis and L. thermophilum, 50 μg/mL; for H. milfordensis, 25 μg/mL. These strains are known to cause fungal infections in aquatic organisms, especially in fishes and mangrove crabs [32]. Hence, it is imperative to search for new antifungal agents against these fungi. The results showed that the antifungal potentials of 1–4 against H. sabahensis were similar to those of other cembranoids: ent-sinuflexibilin D, 14-deoxycrassin, diepoxycembrene A, 5-dehydrosinulariolide, and 11-epi-sinulariolide acetate, except for sinularin, which displayed a lower MIC value [20]. This may be because sinularin possesses α-methylene-δ-lactone and epoxide units. In addition, H. milfordensis was more susceptible to 1–4 than other tested strains.
Experimental
General. The NMR spectra were recorded on a 600-MHz FT-NMR (Jeol, Tokyo, Japan) instrument using CDCl3 with TMS as internal standard. The high-resolution mass spectrum was acquired via LC-ESI-IT-TOF-MS (Shimadzu, Kyoto, Japan). An AUTOPOL IV automatic polarimeter (Rudolph Research Analytical, Hackettstown, USA) was used to measure the optical rotation value at 25°C. Infrared spectra were recorded on a FTIR spectroscopy (Thermo Nicolet, Waltham, USA). Silica gel preparative TLC (Kieselgel 60, F254) and column chromatography (Kieselgel 60, 70–230 mesh) were performed (Merck, Darmstadt, Germany).
Biological Material. The specimen of Sinularia flexibilis was collected from Mantanani Island, Sabah (06°42′4.19′′N, 116°19′58.43′′E) in May 2017. A voucher specimen (BORMI0017) was deposited in the BORNEENSIS Collection of the Institute for Tropical Biology and Conservation, Universiti Malaysia Sabah.
Extraction and Isolation. The fresh soft coral (0.8 kg wet wt) was chopped and extracted with MeOH at room temperature for 5 days. The resulting MeOH extract was concentrated and partitioned between EtOAc and H2O. The EtOAc fraction was further partitioned with hexane and 90% MeOH. The 90% MeOH crude (1.0 g) was subjected to column chromatography eluting with a gradient of hexane–EtOAc (9:1, 8:2, 7:3, 1:1, and 1:0) to yield fractions 1–5. Repeated preparative TLC using CHCl3–EtOAc (95:5) and hexane–EtOAc (9:1) yielded 4 (3.3 mg) from fraction 3 (70.0 mg). Fraction 4 (180.0 mg) was subjected to repeated preparative TLC with toluene–EtOAc (9:1) and CHCl3–EtOAc (9:1) to isolate 2 (5.0 mg), and 3 (9.9 mg), while the residue was further purified by preparative TLC, again using hexane–EtOAc (8:2) to obtain 1 (2.0 mg).
Antifungal Assay. The minimum inhibitory concentration (MIC) of the fungistatic on hyphae was performed by incorporating the pure compound solutions (100, 50, 25, and 12.5 μg/mL) onto PYGS agar in a petri dish followed by inoculation of six tested fungal strains [20, 32]. The MIC was determined visually as the lowest concentration showing no hyphal growth when they were incubated at 25°C for 7 days.
Sinulaflexiolide P (1). Colorless oil; \( {\left[\upalpha \right]}_{\mathrm{D}}^{25} \) –44.0° (c 0.20, CHCl3). IR (KBr, λmax, cm–1): 3400, 1720, 1650, and 1010. 1H NMR (600 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) spectral data (see Table 1). HR-ESI-MS m/z: 387.2156 [M + Na]+ (calcd for C21H32O5Na, 387.2142), 365.2335 [M + H]+ (calcd for C21H33O5, 365.2323), and 347.2210 [M + H – H2O]+ (calcd for C21H31O4, 347.2217).
References
J. W. Blunt, B. R. Copp, R. A. Keyzers, M. H. G. Munro, and M. R. Prinsep, Nat. Prod. Rep., 34, 235 (2017).
J. A. V. Lopez, J. G. Petitbois, C. S. Vairappan, T. Umezawa, F. Matsuda, and T. Okino, Org. Lett., 19, 4231 (2017).
C. Y. Duh, S. C. Chien, P. Y. Song, S. K. Wang, A. A. El-Gamal, and C. F. Dai, J. Nat. Prod., 65, 1853 (2002).
C. S. Phan and C. S. Vairappan, Nat. Prod. Res., 31, 742 (2017).
M. Zhao, J. Yin, W. Jiang, M. Ma, X. Lei, Z. Xiang, J. Dong, K. Huang, and P. Yan, Mar. Drugs, 11, 1162 (2013).
L. F. Liang, L. X. Gao, J. Li, O. Taglialatela-Scafati, and Y. W. Guo, Bioorg. Med. Chem., 21, 5076 (2013).
T. Kamada, C. S. Phan, H. S. Tin, C. S. Vairappan, and T. S. T. Muhammad, Nat. Prod. Commun., 11, 1077 (2016).
P. Yan, Z. Deng, L. van Ofwegen, P. Proksch, and W. Lin, Chem. Pharm. Bull., 58, 1591 (2010).
P. Sun, Q. Yu, J. Li, R. Riccio, G. Lauro, G. Bifulco, T. Kurtan, A. Mandi, H. Tang, T. J. Li, C. L. Zhuang, W. H. Gerwick, and W. Zhang, J. Nat. Prod., 79, 2552 (2016).
B. F. Bowden, B. J. Cusack, and A. Dangel, Mar. Drugs, 1, 18 (2003).
N. E. Awad, M. A. Selim, H. M. Metawe, and A. A. Matloub, Phytother. Res., 22, 1610 (2008).
R. W. Dunlop and R. J. Wells, Aust. J. Chem., 32, 1345 (1979).
L. Li, L. Sheng, C. Y. Wang, Y. B. Zhou, H. Huang, X. B. Li, J. Li, E. Mollo, M. Gavagnin, and Y. W. Guo, J. Nat. Prod., 74, 2089 (2011).
Y. N. Lee, C. J. Tai, T. L. Hwang, and J. H. Sheu, Mar. Drugs, 11, 2741 (2013).
C. H. Cheng, Y. S. Lin, Z. H. Wen, and J. H. Su, Molecules, 17, 10072 (2012).
Y. Li, M. Carbone, R. M. Vitale, P. Amodeo, F. Castelluccio, G. Sicilia, E. Mollo, M. Nappo, G. Cimino, Y. W. Guo, and M. Gavagnin, J. Nat. Prod., 73, 133 (2010).
S. V. Govindam, Y. Yoshioka, A. Kanamoto, T. Fujiwara, T. Okamoto, and M. Ojika, Bioorg. Med. Chem., 20, 687 (2012).
J. H. Sheu, J. H. Su, P. J. Sung, G. H. Wang, and C. F. Dai, J. Nat. Prod., 67, 2048 (2004).
W. T. Chen, Y. Li, and Y. W. Guo, Acta Pharm. Sin. B., 2, 227 (2012).
T. Kamada, C. S. Phan, T. Hamada, K. Hatai, and C. S. Vairappan, Nat. Prod. Commun., 13, 17 (2018).
C. S. Phan, S. Y. Ng, T. Kamada, and C. S. Vairappan, Nat. Prod. Commun., 11, 899 (2016).
A. D. Wright, J. L. Nielson, D. M. Tapiolas, C. H. Liptrot, and C. A. Motti, Mar. Drugs, 10, 1619 (2012).
C. S. Vairappan, I. I. Zanil, T. Kamada, and M. Palaniappan, J. Trop. Biol. Conserv., 9, 200 (2012).
T. Wen, Y. Ding, Z. Deng, L. van Ofwegen, P. Proksch, and W. Lin, J. Nat. Prod., 71, 1133 (2008).
W. T. Chen, J. Li, J. R. Wang, X. W. Li, and Y. W. Guo, RSC Adv., 5, 23973 (2015).
T. C. Tsai, H. Y. Chen, J. H. Sheu, M. Y. Chiang, Z. H. Wen, C. F. Dai, and J. H. Su, J. Agric. Food Chem., 63, 7211 (2015).
L. C. Hu, W. H. Yen, J. H. Su, M. Y. N. Chiang, Z. H. Wen, W. F. Chen, T. J. Lu, Y. W. Chang, Y. H. Chen, W. H. Wang, Y. C. Wu, and P. J. Sung, Mar. Drugs, 11, 2154 (2013).
W. T. Chen, H. L. Liu, and Y. W. Guo, Steroids, 92, 56 (2014).
D. A. Putri, O. K. Radjasa, and D. Pringgenies, Procedia Environ. Sci., 23, 351 (2015).
P. W. Hsieh, F. R. Chang, A. T. McPhail, K. Lee, and Y. C. Wu, Nat. Prod. Res., 17, 409 (2013).
B. N. Ravi and D. J. Faulkner, J. Org. Chem., 43, 2127 (1978).
C. Munchan, K. Hatai, S. Takagi, and A. Yamashita, Aquacult. Sci., 57, 399 (2009).
Acknowledgment
This work was supported by the Sabah Biodiversity Centre (GL0070), the Fujiwara Natural History Foundation, Universiti Malaysia Sabah (GUG0089-SG-2/2016) and the Ministry of Higher Education, Malaysia (FRG0464-2017). The authors would like to thank Prof. Dr. Tatsufumi Okino and Ms. Liang Wei (Hokkaido University) for SciFinder Search as well as Prof. Dr. Hatai (Universiti Malaysia Sabah) for guidance and providing the fungal strains.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2019, pp. 246–248.
Rights and permissions
About this article
Cite this article
Phan, CS., Yee, CS., Vairappan, C.S. et al. Sinulaflexiolide P, A Cembrane-Type Diterpenoid from Bornean Soft Coral Sinularia flexibilis. Chem Nat Compd 55, 285–288 (2019). https://doi.org/10.1007/s10600-019-02668-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-019-02668-w