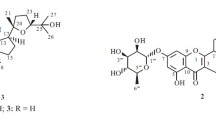
Phytochemical studies on the roots of Euphorbia rapulum afforded a new casbane diterpenoid, 8,12-dihydroxy1βH,2αH-casba-3E,7E,11E-trien-5-one (1), together with four other known compounds, helioscopinolide A (2), 19-hydroxyjolkinolide E (3), jolkinolide E (4), and euphopilolide (5). Their structures were established on the basis of spectroscopic analysis and chemical evidence. All the isolated compounds were evaluated for cytotoxic activities against HepG2, MCF-7, and C6 cell lines, while all compounds showed weak selective activity against all three cell lines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The genus Euphorbia, comprising more than 2000 species, belongs to the family Euphorbiaceae. It is characterized by a toxic and highly skin-irritating latex [1]. Euphorbia has high pharmacological potential, and parts of this genus are used to treat different diseases such as skin disease, gonorrhea, and warts [2, 3]. E. rapulum, a perennial herb of the Euphorbia genus, is primarily distributed in Xinjiang and Central Asia. As far as our literature survey could confirm, only a few studies on E. rapulum have been reported [4]. Therefore, the aim of our study was to evaluate its biologically active compounds. Methanol extracts of E. rapulum were investigated, and we obtained a new casbane diterpenoid, 8,12-dihydroxy-1βH,2αHcasba-3E,7E,11E-trien-5-one (1), together with other four known compounds, helioscopinolide A (2), 19-hydroxyjolkinolide E (3), jolkinolide E (4), and euphopilolide (5). The present paper describes the isolation, structure elucidation, and cytotoxic activity of these compounds.
Compound 1 was isolated as a yellow oil. HR-ESI-MS analysis exhibited a molecular ion peak at m/z 319.2246 [M + H]+, corresponding to a molecular formula of C20H30O3 and implying six degrees of unsaturation. The IR spectrum exhibited bands for hydroxyl and olefinic substituents at 3409 cm–l and 1650 cm–l, respectively. These observations were in agreement with the signals in the 13C NMR spectrum for two secondary oxygenated carbons (δ 71.9 and 74.0), a trisubstituted double bond (δ 150.6 and 135.2), and a ketocarbonyl group (δ 200.4), indicating the presence of an α,β-unsaturated carbonyl group. The 1H NMR spectrum (Table 1) demonstrated resonances of five methyl singlets at δ 1.85 (s, H3-18), 1.41 (s, H3-19), 1.37 (s, H3-20), 1.17 (s, H3-16), and 1.08 (s, H3-17) and five olefinic protons at δ 6.03 (1H, d, J = 10.4 Hz, H-3), 6.05 (1H, d, J = 16.2 Hz, H-6), 6.51 (1H, d, J = 16.2 Hz, H-7), 6.01 (1H, dd, J = 10.8, 15.5 Hz, H-10), and 5.87 (1H, d, J = 15.5 Hz, H-11). Analysis of the 13C NMR spectrum displayed 20 carbon resonances, which were classified by DEPT spectra (Table 1) as six olefinic carbons [δ, 150.6 (C-3), 135.2 (C-4), 127.3 (C-6), 152.1 (C-7), 121.8 (C-10), 141.5 (C-11)] and two hydroxyl carbons [δ 71.9 (C-8) and 74.0 (C-12)]. A detailed analysis of the 2D NMR spectra of 1 allowed the assignment of all proton and carbon atoms. On the basis of 1H–1H COSY corrections, the three structural fragments a (–C-3–C-2–C-1–C-14–), b (–C-9–C-10–C-11–), and c (–C-6–C-7–) (Fig. 1) were established. Since compound 1 has three π-bonds, 1 must be bicyclic to satisfy the six degrees of unsaturation requirement. Besides, the presence of both a quaternary carbon (δ 29.3, C-15) and a gem-dimethyl functionality [δ3 1.17 (s, H3-16), 1.08 (s, H3-17)], together with the HMBC correlation of H3-16/C-1, 2, 15, 17 and H3-17/C-1, 2, 15, 16, indicated a substituted cyclopropyl ring.
Thus, the remaining ring was deduced to be a 14-membered macrocycle, which could be confirmed on the basis of HMBC correlations. The three fragments a–c could be fully connected by inserting quaternary carbons C-4, C-5, C-8, and C-12. At this point, the planar structure of 1 was completed, consistent with a casbane–type diterpenoid [5].
The relative configuration of 1 was established on the basis of the NOESY and 13C NMR experiments (Fig. 1). The E geometry of the double bond Δ3(4) was deduced by the δC values of CH3-18 (< 20 ppm) [6]. Furthermore, the Δ6(7), Δ10(11) was implied as E owing to the coupling constants (J6–7 = 16.2 Hz and J10–11 = 15.5 Hz). There are four chiral centers (C-1, C-2, C-8, and C-12) in the molecule. The correlations between H-1/H3-17, H-1/H-3, and H-2/H3-16, indicated that the junction of the cyclopropyl ring was trans. This assignment was confirmed by the diagnostic 13C NMR chemical shifts of the germinal methyls C-16 and C-17 (δ 21.5 and 24.0), which were significantly different in comparison with those of the co-occurring model compound sinularcasbane K (δC 16.0 and 29.1) [7]. Because of the flexible nature of the 14-membered macrocylic ring, the configuration of C-8 and C-12 could not be decided by the NOESY experiment. Thus, the difference between the structures of 1 and sinularcasbane K was at the configuration of the junction centers (C-1 and C-2). Finally, the structure of compound 1 was elucidated to be 8,12-dihydroxy-1βH,2αH-casba-3E,7E,11E-trien-5-one.
The known compounds were identified as helioscopinolide A (2), 19-hydroxyjolkinolide E (3), jolkinolide E (4), and euphopilolide (5) by comparison with the literature data.
The cytotoxicity of all isolated compounds were evaluated against HepG2, MCF-7, and C6 cancer cell lines by the MTT assay. All compounds showed weak inhibitory activity against all three cell lines [8].
Experimental
General Experimental Procedures. UV spectra were obtained on a Shimadzu UV-2201 spectrophotometer (Shimadzu Co., Tokyo, Japan). IR spectra were recorded on a PerkinElmer 577 spectrophotometer (PerkinElmer Co., Massachusetts, USA). NMR spectra were measured with a Bruker Avance III (HD-400 and 600 MHz) spectrometer in CDCl3 with TMS as internal standard (Bruker, Karlsruhe, Germany). The HR-ESI-MS data were obtained on a Waters LCT Premier XE time-of-flight mass spectrometer (Waters Co., Shanghai, China). Chromatography was performed on silica gel (200–300 mesh; Qingdao Marine Chemical Group, Co., Qingdao, China) and ODS (Octadecylsilyl, 30–50 mm; Tianjin Mical Reagent Co., Tianjin, China), followed by preparative HPLC (Hitachi-L-7110 pump, Hitachi L-7420 UV spectrophotometric detector at 210 nm, YMC C18 reversed-phase column).
Plant Material. Whole plants of Euphorbia rapulum were collected from Ili Kazakh Autonomous Prefecture, Xinjiang Uighur Autonomous Region, China in May 2012. Plants were identified by Dr. Yong Tan (School of Pharmacy, Shihezi University). A voucher specimen (2012050312) has been deposited at the Research Department of Natural Medicine, Shenyang Pharmaceutical University.
Extraction and Isolation. Dried plants of E. rupulum (2.0 kg) were extracted with 95% EtOH three times (20 L, 16 L, 16 L) under reflux conditions, each for 2 h, to yield a crude extract (200.0 g) under reduced pressure. The residue was subjected to silica gel column chromatography (gradient of PE–acetone; 100:1–0:100) to afford fractions 1–8. Fraction 5 (15.0 g) was separated using column chromatography (CC) (ODS, MeOH–H2O; 35–75%) to obtain fractions F1–F5. Purification of fraction F2 (450.0 mg) using CC (ODS, MeOH–H2O 45%), followed by HPLC (CH3CN–H2O, 65:35, flow rate 2.2 mL/min, wavelength 210 nm), yielded 2 (7.5 mg, tR 40 min) and 3 (10.0 mg, tR 47 min). Fraction 8 (29.1 g) was separated using column chromatography (CC) (ODS, MeOH–H2O; 30–80%) to obtain fractions E1–E6. Purification of fraction E2 (300.0 mg) using CC (ODS, MeOH–H2O 40%), followed by HPLC (CH3OH–H2O, 70:30, flow rate 2.0 mL/min, wavelength 210 nm), yielded 1 (4.0 mg, tR 35min). Fraction 1 (3.5 g) was recrystallized to yield 4 (100.0 mg). Fraction 2 (400.0 mg) was recrystallized to yield 5 (15.0 mg).
8,12-Dihydroxy-1βH,2αH-casba-3E,7E,11E-trien-5-one (1), C20H30O3, yellow oil. UV (CHCl3, λmax, nm): 284 (5.53). IR (KBr, νmax, cm–1): 3434, 2921, 1628, 1454, 1384, 1276, 1114. For 1H (400 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) spectral data, see Table 1. HR-ESI-MS m/z 319.2246 [M + H]+ (calcd for C20H31O3, 319.2251).
Helioscopinolide A (2), C20H28O3, white crystals. 1H (600 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) data are as in [9].
19-Hydroxyjolkinolide E (3), C20H28O3, white crystals. 1H (600 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) data are as in [10].
Jolkinolide E (4), C20H28O2, colorless needle crystals. 1H (600 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) data are as in [11].
Euphopilolide (5), C20H28O3, colorless needle crystals. 1H (600 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) data are as in [12].
References
J. Hua, Y. Liu, C. J. Xiao, S. X. Jing, S. H. Luo, and S. H. Li, Phytochemistry, 136, 56 (2017).
Q. W. Shi, X. H. Su, and H. Kiyota, Chem. Rev., 108, 4295 (2008).
L. E. Camargo Luz, A. S. Justo, V. K. Petry, and F. L. Beltrame, J. Ethnopharmacol., 183, 29 (2016).
A. R. Jassbi, Phytochemistry, 67, 1977 (2006).
P. K. Roy, R. Ashimine, H. Miyazato, J. Taira, and K. Ueda, Molecules, 21, 679 (2016).
Y. Li, M. Carbone, R. M. Vitale, P. Amodeo, and F. Castelluccio, J. Nat. Prod., 73, 133 (2010).
B. Yang, J. X. Huang, X. P. Lin, S. R. Liao, and Y. H. Liu, Helv. Chim. Acta, 98, 834 (2015).
T. Mosmann, J. Immunol. Methods, 65, 55 (1983).
B. Y. Zhang, H. Wang, X. D. Luo, Z. Z. Du, J. W. Shen, H. F. Wu, and X. F. Zhang, Phytochemistry, 56, 103 (2001).
G. F. Lai, X. Y. Wang, Y. F. Wang, J. X. Cao, S. D. Luo, and P. Ju, Helv. Chim. Acta, 92, 470 (2009).
N. C. Perellino, L. Garofano, E. Arlandini, and V. Pinciroli, J. Nat. Prod., 59, 773 (1996).
X. D. Zhanga, W. Ni, H. Yan, G. T. Li, H. M. Zhong, Y. Li, and H. Y Liu, Chem. Biodiv., 11, 760 (2014).
Acknowledgment
This work was financially supported by the Key Projects of the National Science and Technology Pillar Program (2012BAI30B02) and the National Natural Science Fund (81260628).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2018, pp. 772–774.
Rights and permissions
About this article
Cite this article
Liu, X.X., Ma, H., He, W. et al. Phytochemical Constituents Isolated from Euphorbia rapulum. Chem Nat Compd 54, 910–912 (2018). https://doi.org/10.1007/s10600-018-2510-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-018-2510-0