Twelve 4-aryl-3,4-dihydrocoumarins were synthesized by the condensation of phenols with the corresponding substituted cinnamic acids in the presence of nitrobenzene and sulfated montmorillonite K-10. This method displayed the property of green chemistry with respect of reuse of the catalyst. These compounds were evaluated for hydroxyl radical scavenging activity in vitro. Results showed that compounds with 7,8-dihydroxy groups had relatively strong hydroxyl radical scavenging activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Natural compounds with the 4-arylcoumarin structure have diverse biological activities and provide a platform for chemical modification [1]. Just as Ajit Kumar and co-workers have verified, 4-aryl-3,4-dihydrocoumarins have similar activity, with 4-arylcoumarins as its dihydro analogs [2]. Notably, several investigators have demonstrated that 4-aryl-3,4-dihydrocoumarins that possess two hydroxyl groups in ortho position exhibit potent antioxidant property [3, 4]. However, the free radicals they used for evaluating the antioxidant activity of 4-aryl-3,4-dihydrocoumarins were limited to DPPH, ABTS+, and NO. In this paper, we focus on its hydroxyl radical scavenging activity. In addition, this kind of compounds also have estrogen-like activity [5], immunomodulatory activity [6], and antiaging activity [7]. Hence, as part of our further study of 4-aryl-3,4-dihydrocoumarins, we report the synthesis and antioxidant activity of twelve 4-aryl-3,4-dihydrocoumarins.
The most common method for the synthesis of 4-aryl-3,4-dihydrocoumarins is the Ponndorf reaction of phenols with substituted cinnamic acids promoted by acids including H2SO4, HCl, and PPA, Lewis acid, or clay [8]. For example, p-toluenesulfonic acid mediates hydroarylation of cinnamic acids with phenols under metal and solvent-free conditions [9], PPA mediates condensation of cinnamic acids with phenols [10], condensation of resorcinol and methylcinnamate in the presence of anhydrous AlCl3 and HCl [11], condensation of phenols and cinnamic acids, in particular with hydroxy and methoxy substituents in the presence of CF3COOH or BF3–Et2O and POCl3 [12, 13], with or without microwave-assisted synthesis from phenols and cinnamoyl chloride in the presence of montmorillonite K-10 catalyst [3, 14], and recently solvent-free synthesis of 4-aryl-3,4-dihydrobenzopyran-2-ones via [3+3] cyclocoupling of phenols with cinnamic acid catalyzed by molecular iodine [15]. However, most of these methods involve relatively harsh reaction conditions, low functional group tolerance, severe environmental pollution, or difficulty in separation and purification of products. Hence their applications have been somewhat constrained. In this article, we disclosed another methodology for the synthesis of 4-aryl-3,4-dihydrocoumarins based on sulfated montmorillonite K-10.
In spite of the synthesis of some 4-aryl-3,4-dihydrocoumarins successfully catalyzed by montmorillonite K-10, these reactions usually needed long reaction times, tedious isolation and purification processes, and used phenols and cinnamoyl chloride as reactants. To the best of our knowledge, the conversion of cinnamic acid to cinnamoyl chloride required absolutely anhydrous conditions that added difficulty to the operation in the experiment. Moreover, crude products are usually purified by chromatography on silica gel, leading to waste of eluent and pollution of the environment. In our experiment, we used phenols and substituted cinnamic acids as initial reactants and crystallization to purify the crude products. What is more, the montmorillonite K-10 that was used as catalyst for synthesizing certain 4-aryl-3,4-dihydrocoumarin can be applied to synthesize the same compound without extra treatment.
Twelve 4-aryl-3,4-dihydrocoumarins 9–20 were synthesized by the condensation of phenols 1–4 with the corresponding substituted cinnamic acids 5–8 in the presence of nitrobenzene and sulfated montmorillonite K-10, resulting in 52.2–83.3% yields (Scheme 1). Before its use as a high-efficient catalyst, commercially available montmorillonite K-10 was treated in 30% H2SO4 solution for 4 h under 95°C in a three-neck round-bottom flask. This treatment method [16] was used with slight modification.

Scheme 1.
The structures of all the synthesized compounds were supported by FT-IR, MS, 1H NMR, and 13C NMR spectral data.
We have also examined the reuse of the catalyst on the tandem esterification alkylation of substituted cinnamic acids and resorcinol to get dihydrocoumarin 9. The results shown in Table 1 indicate that another advantage of montmorillonite K-10 lies in the fact that it can be reused without extra treatment, together with the cheapness, nontoxicity, and commercial availability of this clay, rendering the synthetic process more environmentally friendly and economic.
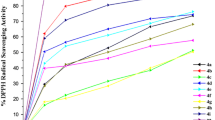
All the synthesized compounds were evaluated for their antioxidant activities by way of scavenging hydroxyl radicals. The hydroxyl radical scavenging activity of the twelve 4-aryl-3,4-dihydrocoumarins, as indicated by the half maximal inhibitory concentration (IC50) values, are listed in Table 2. The results showed that 4-aryl-3,4-dihydrocoumarins with 7,8-dihydroxy groups had relatively strong hydroxyl radical scavenging activity, which was a little weaker than vitamin C, while the other compounds presented little or no ability to scavenge hydroxyl radical, even at the highest test concentration of 2.00 mg·mL–1. Notably, compound 14 showed the highest activity, with an IC50 value of 0.91 ± 0.03 mg·mL–1 (Table 2).
In conclusion, twelve 4-aryl-3,4-dihydrocoumarins were synthesized using sulfated montmorillonite K-10 as catalyst. This method possesses such advantages as being more environmental friendly, and more sustainable and economic, requiring simple isolation and purification processes, producing few byproducts, the use of earth-abundant catalysts, and producing relativity high yields. With respect to their antioxidant activity, compounds 12, 13, 14, and 15 have strong hydroxyl radical scavenging activity.
Experimental
General. Melting points were determined using a Thiele tube and were uncorrected. The FT-IR spectra were recorded using a Thermo-Nicolet Nexus 670 spectrometer with KBr pellets. The 1H NMR and 13C NMR spectra were recorded with a Bruker AM-600 spectrometer with TMS as the internal standard. Chemical shifts are reported in ppm with DMSO-d6 as solvent. Spin multiplets are given as s (singlet), d (doublet), t (triplet), q (quartet), and m (multiplet). Mass spectra were obtained with an Agilent Trap VL LC/MS spectrometer. The absorbance were recorded by a Hitachi U-3000 UV spectrophotometer.
Treatment of Montmorillonite K-10. The montmorillonite K-10 used in this study was supplied by Shanghai Aladdin Bio-Chem. To a 250 mL oven-dried three-neck round-bottom flask, 13 g montmorillonite K-10 was mixed with 130 mL 30% H2SO4 solution. The suspension was heated to 95°C and kept for 4 h under magnetic stirring. Then the acid-treated clays were washed thoroughly with distilled water until the pH of the washings was 7. The samples were dried in a vacuum oven at 40°C to get 13.95 g sulfated montmorillonite K-10.
General Procedure for the Synthesis of 4-Aryl-3,4-dihydrocoumarins 9–20. A mixture of a substituted cinnamic acid (5 mmol) and a substituted phenol (5 mmol) in nitrobenzene (10 mL) was heated to 100°C. Sulfated montmorillonite K-10 (2 g) was added to the mixture after all reagents were dissolved, and the mixture was stirred at 100°C for 3–12 h. The reaction process was monitored by TLC (CH2Cl2–CH3OH, 10:1). The suspension was directly filtered, and a suitable amount of petroleum ether was added to the filtrate. Then the mixture was cooled and stored or stirred under low temperature for a certain length of time to promote crystallization of the product. The crude product was recrystallized from EtOAc–PE to afford 9–20.
(±)-7-Hydroxy-4-(4-methoxyphenyl)-3,4-dihydrocoumarin (9). White solid, mp 177.2–178.3°C, yield 79.2%. IR (KBr, ν, cm–1): 3332 (OH), 3040, 2800 (OCH3), 1736 (C=O), 1614, 1583, 1507, 1450, 1236, 1146 (C–O), 1105, 1028, 984, 832 (Ar). PMR spectrum (600 MHz, DMSO-d6, δ, ppm, J/Hz): 3.03 (2H, m, H-3), 3.73 (3H, s, 4′-OCH3), 4.32 (1H, t, J = 6.0, H-4), 6.53 (1H, dd, J = 2.4, 8.4, H-6), 6.55 (1H, d, J = 2.4, H-8), 6.84 (1H, d, J = 8.5, H-5), 6.90 (2H, d, J = 8.8, H-3′, 5′), 7.06 (2H, d, J = 8.6, H-2′, 6′), 9.74 (1H, s, 7-OH). 13C NMR (150 MHz, DMSO-d6, δ, ppm): 36.76, 37.80, 54.95, 103.17, 111.51, 114.03, 116.47, 128.23, 128.81, 133.53, 151.81, 157.43, 158.10, 167.73. MS m/z: 270.7 [M + 1]+, 162.6.
(±)-7-Hydroxy-4-(3,4-dimethoxyphenyl)-3,4-dihydrocoumarin (10). White solid, mp 144.8–146.1°C, yield 53.3%. IR (KBr, ν, cm–1): 3433 (OH), 3030, 2790 (OCH3), 1767 (C=O), 1628, 1597, 1514, 1447, 1267, 1142 (C–O), 1101, 1025, 848, 810 (Ar). PMR spectrum (600 MHz, DMSO-d6, δ, ppm, J/Hz): 3.05 (2H, m, H-3), 3.70 and 3.71 (each 3H, s, 3′, 4′-OCH3), 4.29 (1H, t, J = 6.0, H-4), 6.52 (1H, s, H-8), 6.53–6.56 (2H, m, H-6′, 5′), 6.83–6.89 (3H, m, H-6, 2′, 5), 9.74 (1H, s, 7-OH). 13C NMR (150 MHz, DMSO-d6, δ, ppm): 37.27, 38.81, 55.93, 55.95, 103.72, 111.76, 112.09, 112.34, 119.54, 129.40, 149.37, 158.00, 168.37. MS m/z: 300.8 [M + 1]+, 258.7, 190.6, 162.7.
(±)-7-Hydroxy-4-(3-methoxy-4-hydroxyphenyl)-3,4-dihydrocoumarin (11). White solid, mp 194.3–196.3°C, yield 54.9%. IR (KBr, ν, cm–1): 3415 (OH), 2994, 2929 (OCH3), 1724 (C=O), 1625, 1602, 1518, 1454, 1259, 1152 (C–O), 1112, 1028, 841, 809 (Ar). PMR spectrum (600 MHz, DMSO-d6, δ, ppm, J/Hz): 3.03 (2H, m, H-3), 3.71 (3H, s, 3′-OCH3), 4.24 (1H, t, J = 6.0, H-4), 6.44 (1H, d, J = 7.8, H-6), 6.51 (1H, s, H-8), 6.54 (1H, d, J = 8.4, H-6′), 6.70 (1H, d, J = 8.1, H-5′), 6.78 (1H, s, H-2′), 6.84 (1H, d, J = 7.8, H-5), 8.93 and 9.72 (each 1H, s, 4′, 7-OH). 13C NMR (151 MHz, DMSO-d6, δ, ppm): 37.35, 38.81, 56.05, 103.69, 112.05, 115.93, 117.23, 119.84, 129.42, 132.94, 146.01, 148.20, 152.37, 157.95,168.45. MS m/z: 287.1 [M + 1]+, 162.8.
(±)-7,8-Dihydroxy-4-(4-methoxyphenyl)-3,4-dihydrocoumarin (12). White solid, mp 168.9–171.1°C, yield 79.4%.IR (KBr, ν, cm–1): 3330 (OH), 3050, 2805 (OCH3), 1738 (C=O), 1634, 1612, 1512, 1458, 1236, 1154 (C–O), 1109, 1030, 1002, 830, 802 (Ar). PMR spectrum (400 MHz, DMSO-d6, δ, ppm, J/Hz): 3.01 (2H, m, H-3), 3.72 (3H, s, 4′-OCH3), 4.28 (1H, t, J = 6.8, H-4), 6.28 (1H, d, J = 8.0, H-6), 6.53 (1H, d, J = 8.0, H-5), 6.88 and 7.06 (each 2H, d, J = 8.0, H-2′, 6′, 3′, 5′), 8.91 and 9.29 (each 1H, s, 7, 8-OH). 13C NMR (150 MHz, DMSO-d6, δ, ppm): 37.35, 38.93, 55.53, 111.7, 114.9, 118.5, 119.1, 128.83, 133.74, 134.11, 140.99, 146.31, 158.66, 168.25. MS m/z: 286.8 [M + 1]+, 244.6, 178.6, 160.6.
(±)-7,8-Dihydroxy-4-(3,4-dimethoxyphenyl)-3,4-dihydrocoumarin (13). White solid, mp 173.7–174.6°C, yield 83.3%. IR (KBr, ν, cm–1): 3423 (OH), 2935, 2829 (OCH3), 1754 (C=O), 1610, 1515, 1470, 1413, 1240, 1137 (C–O), 1025, 807 (Ar). PMR spectrum (600 MHz, DMSO-d6, δ, ppm, J/Hz): 3.04 (2H, m, H-3), 3.70 and 3.71 (each 3H, s, 3′, 4′-OCH3), 4.27 (1H, t, J = 6.3, H-4), 6.28, 6.53, 6.56, and 6.87 (each 1H, d, J = 8.2, H-6′, 5′, 6, 5), 6.84 (1H, s, H-2′), 8.90 and 9.28 (each 1H, s, 7, 8-OH). 13C NMR (150 MHz, DMSO-d6, δ, ppm): 37.25, 39.98, 55.96, 111.48, 111.87, 112.32, 117.61, 118.26, 119.62, 133.68, 134.56, 140.99, 146.28, 148.26, 149.32, 168.34. MS m/z: 316.8 [M + 1]+, 178.5.
(±)-7,8-Dihydroxy-4-(3-methoxy-4-hydroxyphenyl)-3,4-dihydrocoumarin (14). White solid, mp 171.7–173.1°C, yield 53.1%. IR (KBr, ν, cm–1): 3373 (OH), 3018, 2810 (OCH3), 1743 (C=O), 1612, 1517, 1464, 1274, 1067 (C–O), 1033, 812, 693 (Ar). PMR spectrum (600 MHz, DMSO-d6, δ, ppm, J/Hz): 3.01 (2H, m, H-3), 3.71 (3H, s, 3′-OCH3), 4.22 (1H, t, J = 6.2, H-4), 6.29 (1H, d, J = 8.3, H-6), 6.45 (1H, dd, J = 8.1, 1.5, H-6′), 6.52 (1H, d, J = 8.1, H-5), 6.69 (1H, d, J = 8.1, H-5′), 6.78 (1H, d, J = 1.5, H-2′), 8.88, 8.90, and 9.26 (each 1H, s, 7, 8, 4′-OH). 13C NMR (150 MHz, DMSO-d6, δ, ppm): 37.34, 39.98, 56.08, 111.45, 112.19, 115.88, 117.63, 118.45, 119.90, 132.95, 133.65, 140.96, 145.97, 146.21, 148.14, 168.41. MS m/z: 303.1 [M + 1]+, 283.7, 260.7, 178.7, 150.6.
(±)-7,8-Dihydroxy-4-(3-hydroxy-4-methoxyphenyl)-3,4-dihydrocoumarin (15). White solid, mp 185.0–187.1, yield 66.4%. IR (KBr, ν, cm–1): 3499, 3420 (OH), 3010, 2810 (OCH3), 1752 (C=O), 1610, 1591, 1518, 1469, 1277, 1068 (C–O), 1030, 786, 698 (Ar). PMR spectrum (600 MHz, DMSO-d6, δ, ppm, J/Hz): 2.97 (2H, m, H-3), 3.72 (3H, s, 4′-OCH3), 4.19 (1H, t, J = 5.5, H-4), 6.32 (1H, d, J = 8.2, H-6), 6.53 (3H, m, H-5, 6′, 2′), 6.84 (1H, d, J = 8.2, H-5′), 8.90, 8.95, and 9.29 (each 1H, s, 7, 8, 3′-OH). 13C NMR (150 MHz, DMSO-d6, δ, ppm): 37.44, 39.11, 56.10, 111.47, 112.85, 114.92, 117.73, 118.25, 133.68, 134.92, 140.96, 146.23, 147.03, 168.26. MS m/z: 302.9 [M + 1]+, 283.7, 260.7, 178.6, 150.6.
(±)-5,7-Dihydroxy-4-(4-methoxyphenyl)-3,4-dihydrocoumarin (16). White solid, mp 148.4–149.9°C, yield 71.6%. IR (KBr, ν, cm–1): 3510 (OH), 3050, 2805 (OCH3), 1759 (C=O), 1635, 1611, 1513, 1457, 1231, 1179 (C–O), 1134, 1061, 1032, 826 (Ar). PMR spectrum (400 MHz, DMSO-d6, δ, ppm, J/Hz): 2.97 (2H, m, H-3), 3.69 (3H, s, 4′-OCH3), 4.38 (1H, d, J = 6.5, H-4), 6.02 (1H, d, J = 2.1, H-6), 6.17 (1H, d, J = 2.2, H-8), 6.83 and 6.98 (each 2H, d, J = 8.6, H-3′, 5′, and 2′, 6′), 9.55 and 9.73 (each 1H, s, 5, 7-OH). 13C NMR (150 MHz, DMSO-d6, δ, ppm): 33.40, 37.75, 61.07, 94.51, 95.15, 99.18, 103.88, 114.44, 128.13, 134.74, 153.38, 155.79, 158.28, 168.41. MS m/z: 286.9 [M + 1]+, 178.7, 110.8.
(±)-5,7-Dihydroxy-4-(3,4-dimethoxyphenyl)-3,4-dihydrocoumarin (17). White solid, mp 185.8–187.7°C, yield 61.8%. IR (KBr, ν, cm–1): 3402 (OH), 1778 (C=O), 1632, 1610, 1500, 1460, 1237, 1148 (C–O), 1012, 825 (Ar). PMR spectrum (600 MHz, DMSO-d6, δ, ppm, J/Hz): 3.02 (2H, m, H-3), 3.68 and 3.70 (each 3H, s, 3′, 4′-OCH3), 4.37 (1H, d, J = 6.7, H-4), 6.01 (1H, s, H-6), 6.17 (1H, s, H-8), 6.40 (1H, dd, J = 8.3, H-6′), 6.81 (1H, d, J = 8.3, H-5′), 6.81 (1H, d, J = 1.5, H-2′), 9.56 and 9.74 (each 1H, s, 5, 7-OH). 13C NMR (151 MHz, DMSO-d6, δ, ppm): 33.76, 37.74, 55.87, 55.95, 95.11, 99.17, 103.74, 111.49, 112.22, 118.39, 135.29, 148.08, 153.45, 155.81, 158.27, 168.47. MS m/z: 316.9 [M + 1]+, 178.7, 164.7, 110.8.
(±)-5,7-Dihydroxy-4-(3-methoxy-4-hydroxyphenyl)-3,4-dihydrocoumarin (18). White solid, mp 173.0–174.0°C, yield 53.1%. IR (KBr, ν, cm–1): 3404 (OH), 3030, 2790 (OCH3), 1767 (C=O), 1628, 1597, 1514, 1447, 1267, 1142 (C–O), 1101, 1025, 848, 810 (Ar). PMR spectrum (600 MHz, DMSO-d6, δ, ppm, J/Hz): 3.01 (2H, m, H-3), 3.70 (3H, s, 3′-OCH3), 4.33 (1H, d, J = 6.7, H-4), 6.00 (1H, s, H-6), 6.16 (1H, s, H-8), 6.30 (1H, d, J = 8.1, H-6′), 6.62 (1H, d, J = 8.1, H-5′), 6.75 (1H, s, H-2′), 8.91, 9.54, and 9.72 (each 1H, s, 5, 7, 4′-OH). 13C NMR (150 MHz, DMSO-d6, δ, ppm): 33.73, 37.86, 56.00, 95.09, 99.16, 103.95, 111.78, 115.72, 118.78, 133.68, 145.78, 148.05, 153.42, 155.78, 158.19, 168.55. MS m/z: 303.1 [M + 1]+, 261.1, 179.1, 151.1, 111.2.
(±)-6-Hydroxy-4-(4-methoxyphenyl)-3,4-dihydrocoumarin (19). White solid, mp 167.7–169.7°C, yield 52.2%. IR (KBr, ν, cm–1): 3332 (OH), 3000, 2829 (OCH3), 1728 (C=O), 1613, 1600, 1502, 1450, 1236, 1146 (C–O), 1105, 1028, 831, 807 (Ar). PMR spectrum (600 MHz, DMSO-d6, δ, ppm, J/Hz): 3.01 (2H, m, H-3), 3.73 (3H, s, 4′-OCH3), 4.34 (1H, t, J = 6.3, H-4), 6.37 (1H, s, H-5), 6.68 (1H, d, J = 8.6, H-7), 6.92 (2H, d, J = 8.0, H-3′, 5′), 6.96 (1H, d, J = 8.6, H-8), 7.10 (2H, d, J = 8.0, H-2′, 6′), 9.34 (1H, s, 6-OH). 13C NMR (150 MHz, DMSO-d6, δ, ppm): 36.8, 39.99, 55.55, 114.68, 115.23, 117.76, 128.05, 129.05, 133.34, 144.43, 154.37, 158.81, 168.53. MS m/z: 207.7 [M + 1]+, 162.6.
(±)-6-Hydroxy-4-(3,4-dimethoxyphenyl)-3,4-dihydrocoumarin (20). White solid, mp 166.7–168.5°C, yield 53.3%. IR (KBr, ν, cm–1): 3259 (OH), 3071, 2790 (OCH3), 1709 (C=O), 1593, 1522, 1487, 1451, 1267, 1142 (C–O), 1106, 1022, 856, 817 (Ar). PMR spectrum (600 MHz, DMSO-d6, δ, ppm, J/Hz): 3.04 (2H, m, H-3), 3.72 and 3.73 (each 3H, s, 3′, 4′-OCH3), 4.33 (1H, t, J = 6.3, H-4), 6.37 (1H, d, J = 2.6, H-5), 6.62 (1H, dd, J = 1.6, 8.2, H-6′), 6.68 (1H, dd, J = 2.7, 8.7, H-7), 6.88 (1H, d, J = 1.5, H-2′), 6.91 (1H, d, J = 8.3, H-5′), 6.96 (1H, d, J = 8.7, H-8), 9.32 (1H, s, 6-OH). 13C NMR (150 MHz, DMSO-d6, δ, ppm): 36.70, 39.99, 55.90, 111.99, 112.43, 114.67, 115.22, 117.70, 119.88, 128.06, 133.72, 144.42, 148.42, 149.41, 154.35, 168.59. MS m/z: 301.0 [M + 1]+, 259.0, 162.9.
Hydroxyl Radical Scavenging Activity Assay. The ability of 9–20 and vitamin C to scavenge the hydroxyl radical was evaluated with method as described by Cai et al. [17] with slight modifications. In a test tube 1 mL H2O2 solution (0.03%), 1 mL FeSO4 solution (2 mmol·L–1), 1 mL salicylic acid solution (10 mmol·L–1), and 1 mL of 4-aryl-3,4-dihydrocoumarins and vitamin C with different concentrations (0.4–2.0 mg/mL) were mixed. Then 1 mL H2O2 solution (0.03%) was added to this mixture to start the reaction. The solutions were kept at 37°C. After 30 min, absorbance was measured at 510 nm against a reagent blank which use distilled water to replace the H2O2 solution. The percentage of OH scavenging by 9–20 and vitamin C was calculated using the following equation:
where A0 is the absorbance of 1.0 mL distilled water + 1.0 mL H2O2 + 1 mL FeSO4 + 1 mL salicylic acid + 1.0 mL H2O2; A1 is the absorbance of 1.0 mL 4-aryl-3,4-dihydrocoumarins or standard + 1.0 mL H2O2 + 1.0 mL FeSO4 + 1.0 mL salicylic acid + 1.0 mL H2O2; A2 is the absorbance of 1.0 mL 4-aryl-3,4-dihydrocoumarins or standard + 1.0 mL H2O2 +1.0 mL FeSO4 + 1.0 mL absolute ethyl alcohol + 1.0 mL H2O2.
References
M. M. Garazd, Y. L. Garazd, and V. P. Khilya, Chem. Nat. Compd, 39, 54 (2003).
A. Kumar, B. K. Singh, R. Tyagi, S. K. Jain, S. K. Sharma, A. K. Prasad, H. G. Raj, R. C. Rastogi, A. C. Wattersonc, and V. S. Parmar, Bioorg. Med. Chem., 13, 4300 (2005).
J. M. Lee, T. H. Tseng, and Y. J. Lee, Synthesis, 15, 2247 (2001).
K. Y. Zhang, W. X. Ding, J. Sun, B. Zhang, F. J. Lu, R. Lai, Y. Zou, and G. Yedid, Biochimie, 107, 203 (2014).
F. Roelens, K. Huvaere, W. Dhooge, M. V. Cleemput, F. Comhaire, and D. D. Keukeleire, Eur. J. Med. Chem., 40, 1042 (2005).
X. F. Zhang, H. M. Wang, Y. L. Song, L. H. Nie, L. F. Wang, B. Liu, P. P. Shen, and Y. Liu, Bioorg. Med. Chem. Lett., 16, 949 (2006).
J. Posakony, M. Hirao, S. Stevens, J. A. Simon, and A. Bedalov, J. Med. Chem., 47, 2635 (2004).
M. M. Garazd, Y. L. Garazd, and V. P. Khilya, Chem. Nat. Compd., 41, 245 (2005).
A. R. Jagdale and A. Sudalai, Tetrahedron Lett., 48, 4895 (2007).
P. L. Majumder, J. Ind. Chem. Soc., 78, 743 (2001).
A. K. D. Gupta, K. R. Das, and A. D. Gupta, Ind. J. Chem., 10, 32 (1972).
E. Rizzi, S. Dallavalle, and L. Merlini, Synth. Commun., 36, 1117 (2006).
J. Sun, W. X. Ding, X. P. Hong, K. Y. Zhang, and Y. Zou, Chem. Nat. Compd., 48, 16 (2012).
Z. Zhang, Y. Ma, and Y. F. Zhao, Synlett, 7, 1091 (2008).
D. P. Kamat, S. G. Tilve, and V. P. Kamat, Tetrahedron Lett., 53, 4469 (2012).
C. N. Rhodes and D. R. Brown, Catal. Lett., 24, 285 (1994).
B. Q. Cai, Z. Y. Cai, F. D. Zhang, and M. J. Hang, Acta Agric. Univ. Jingxiensis, 32, 813 (2010).
Acknowledgment
This work was financially supported by the Project of Shandong Province Higher Educational Science and Technology Program (J14M02), the Science and Technology Research Program of Shandong Academy of Medical Sciences (2014-4), and Shandong Provincial Natural Science Foundation (ZR2015YL041).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2017, pp. 733–736.
Rights and permissions
About this article
Cite this article
Li, N., Wang, B., Sun, Jy. et al. Synthesis and Hydroxyl Radical Scavenging Activity of 4-Aryl-3,4-Dihydrocoumarins. Chem Nat Compd 53, 860–865 (2017). https://doi.org/10.1007/s10600-017-2141-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-2141-x