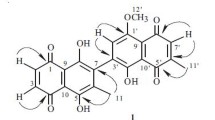
A new dimeric naphthoquinone, named 5,8,5 ′ -trihydroxy-8 ′ -methoxy-6,6 ′ -dimethyl-7,3-binaphthyl-1,4,1 ′ ,4 ′ -tetraone ( 1 ), was isolated from Diospyros lotus stem. The structure of the compound was elucidated by advance spectroscopic analysis including 1D and 2D experiments such as HMBC, NOESY, and J-resolved (JRES). Compound 1 was assessed for antioxidant activity, which showed good activity even at low concentration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Diospyros lotus belongs to the family Ebenaceae, prevalent in subtropical and tropical regions. Different parts of the genus Diospyros are used as medicinal remedies [1]. The extracts and derived compounds of D. lotus have been studied for their antioxidant, anti-inflammatory, antimicrobial, muscle relaxant, and sedative activities [2,3,4,5,6]. Our research group has empirically validated some of the traditional uses of D. lotus collected from Pakistan. Phytochemical profiling of D. lotus extract showed the presence of an array of bioactive secondary metabolites. The current finding deals with the isolation, structure elucidation, and antiradical potential of a new dimeric naphthoquinone (1) from the plant extract.
Compound 1 (mp 254–256°C) was purified as a reddish amorphous powder from the chloroform fraction of D. lotus. Its EI-MS exhibited a molecular ion peak at m/z 420.4 consistent with the molecular formula C23H16O8. The IR spectrum showed absorption bands at 3646 for an OH group, 2985 cm–1 for a CH group, 1650 cm–1 for a conjugated CO group, and 1618, 1605, and 1464 cm–1 for a CH aromatic moiety. The UV spectrum exhibited absorptions at 256, 294, and 434 nm. The assignment of protons and carbons was carried out by HMBC, HMQC, 1H–1H COSY, and J-resolved experiments (Table 1). The types of carbons were identified by 13C NMR (BB, DEPT). The 1H NMR spectrum of compound 1 revealed two tertiary methyl groups [δ 1.98, 2.01 (each 3H, s)], one methoxy moiety at 3.61 (3H, s), three quinoid protons H-2 [δ 6.55 (d, J = 10.5 Hz), H-3 [δ 6.91 (d, J = 10.5 Hz)], and H-2′ (δ 7.72, s), and one aromatic proton H-7′ (δ 6.71, s). The 13C NMR and DEPT spectra (Table 1) exhibited 23 carbons signals for two methyl carbons, one methoxy carbon and 20 carbons for two naphthoquinone units. The substituents were placed with the help of HMBC correlations as shown in Fig. 1.
Compound 1 shows significant quenching effect on the free radical in a dose-dependent manner with percent activity of 84.11% at 100 μg/mL. The compound showed IC50 of 40.21 0.90 μg/mL against vitamin C (IC50 12.18 0.77 μg/mL).
Experimental
General Procedure. The melting point of compound 1 was determined in BioCote (Bibby Scientific limited). UV of compound 1 was measured using a Hitachi-U-3200 UV-visible spectrometer. IR spectrum was recorded on an FT-IR (Nicolet 380; Thermo Scientific) spectrometer. 1H (600 MHz) and 13C NMR (150 MHz) spectra were recorded on an Avance AV-600 Cryoprob NMR instrument in CDCl3. HR-EI-MS were measured on a JEOL JMS 600H mass spectrometer; EI source 70 eV. Normal phase column chromatography (CC) was performed using silica gel 60 (0.062–0.200 mm; Merck). TLC was run using aluminum plates precoated with silica gel 60 (F254; Merck).
Plant Material. The root samples of D. lotus were collected from Dir district of KPK Province, Pakistan in July 2015. The specimen was identified by Prof. Dr. Abdur Rashid from the Department of Botany, University of Peshawar, Pakistan. A voucher specimen (Bot. 20036(PUP) has been deposited in the Department.
Extraction and Isolation. The shade-dried roots fragments of D. lotus (4 kg) were pulverized and repeatedly extracted with chloroform at room temperature. The extracts were concentrated by evaporating the solvents under reduced pressure at a temperature below 55°C using a rotary evaporator to obtain a dark red residue (67 g). The crude extract was defatted with hexane through a Soxhlet apparatus to remove color and dye [7,8,9]. Ten grams of the defatted extract was subjected to column chromatography using silica gel. The column was eluted with n-hexane–ethyl acetate (100:0→0:15→0:100) as the solvent system. A total of 50 fractions was obtained based on TLC profiles. Fractions 10–15 were pooled based on their TLC profile and were subjected to pencil column chromatography using n-hexane–ethyl acetate (100:0→12:100), which yielded a new compound, 5,8,5′-trihydroxy-8′-methoxy-6,6′-dimethyl-7,3-binaphthyl-1,4,1′,4′-tetraone (1). Identification of compound 1 was conducted by advanced 2D NMR.
DPPH Free Radical Scavenging Assay. The antioxidant activity was studied using the DPPH radical scavenging assay according to standard protocol as discussed earlier [10]. Hydrogen atom or electron donating abilities of the corresponding fractions and standards were measured from the bleaching of the purple-colored methanol solution of DPPH. For this, 1 mM of methanol-based DPPH radical solution was prepared, of which 1 mL was mixed with 3 mL of methanol-based sample solutions. The concentration of the samples varied with fractions, pure compounds, and control containing 10–100 μg/mL, 5–100 μg, and no sample, respectively. The solution was incubated for 30 min in the dark, followed by absorbance measurements at 517 nm.
The decrement of the DPPH solution absorbance indicated the increase in the DPPH radical-scavenging activity. The percent radical scavenging activity (%RSA) was calculated as follows:
where ODcontrol is the absorbance of the blank sample, and ODsample is the absorbance of the sample. All the experiments were carried out in triplicate.
References
G. Uddin, A. Rauf, B. S. Siddiqui, and S. Q. Shah, Middle East J. Sci. Res., 1, 78 (2011).
G. Uddin, A. Rauf, B. S. Siddiqui, N. Muhammad, A. Khan, and S. U. A. Shah, Phytomedicine, 21, 7 (2014).
M. Tezuka, C. Takahashi, M. Kuroyanagi, M. Satake, K. Yoshihira, and S. Natori, Phytochemistry, 12, 175 (1973).
Abdur Rauf, Ghias Uddin, Abdul Latif, and Naveed Muhammad, Chem. Nat. Compd., 50, 97 (2014).
J. M. Watt and M. G. Breyer-Brandwijk, The Medicinal and Poisonous Plants of Southern Africa, E. & S. Livingstone, Edinburgh, 1932.
R. N. Chopra and S. L. Nayar, Glossary of Indian Medicinal Plants, Council of Scientific and Industrial Research, New Delhi, 1956.
A. Rauf, G. Uddin, B. S. Siddiqui, A. Khan, H. Khan, M. Arfan, and M. A. Wadood, Phytomedicine, 21 (12), 1509 (2014).
A. Rauf, G. Uddin, B. S. Siddiqui, N. Muhammad, and H. Khan, Asian Pac. J. Trop. Biomed., Suppl. 1, S382 (2014).
G. Uddin, A. Rauf, M. Arfan, I. Khan, M. Ali, M. Taimur, and I. ur-Rehman, J. Enzyme Inhib. Med. Chem., 27 (5), 646 (2012).
A. Rauf, G. Uddin, H. Khan, and R. Ullah, Pak. J. Pharm. Sci., 27, 4 (2014).
Acknowledgment
The authors would like to thank the Institute of Scirntific Research and Revival of Islamic Heritage, Deanship of Scientific Research at Umm Al-Qura University (Project ID 43310005) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2017, pp. 725–726.
Rights and permissions
About this article
Cite this article
Rauf, A., Hadda, T.B., Patel, S. et al. Identification, Structure Elucidation, and Antioxidant Potential of a New Compound from Diospyros lotus . Chem Nat Compd 53, 849–851 (2017). https://doi.org/10.1007/s10600-017-2138-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-2138-5