A new benzenoid, propyl 7-methoxybenzo[d][1,3]dioxole-5-carboxylate (1), has been isolated from the stems of Capparis acutifolia, together with five known compounds, rutin (2), 3β-hydroxystigmast-5-en-7-one (3), 6β-hydroxystigmast-4-en-3-one (4), sitosterol trans-ferulate (5), and sitosterol cis-ferulate (6). The structure of the new compound 1 was determined through spectroscopic and MS analyses. Rutin (2) exhibited potent inhibition, with IC50 value of 1.26 ± 0.15 μg/mL, against formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP)-induced superoxide anion (O2 ·–) generation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Capparis acutifolia Sweet (Capparaceae) is a small shrub, distributed throughout southern China, Vietnam, the Ryukyus, and Taiwan [1]. Flavonoids [2, 3, 5], benzenoids [2, 6], gallotannins [4], anthraquinones [6], and their derivatives are widely distributed in plants of the genus Capparis. Many of these compounds exhibit diverse biological activities, including anti-H5N1 virus [3], insulinomimetic [4], and hepatoprotective [5] activities. In a preliminary screening, the methanolic extract of the stems of this species showed anti-inflammatory activity in vitro. The current phytochemical investigation of the stems of this plant has led to the isolation of a new benzenoid, propyl 7-methoxybenzo[d][1,3]dioxole-5-carboxylate (1), along with five known compounds. The structural elucidation of 1 and anti-inflammatory property of 2 are described herein.
Extensive fractionation of the CH2Cl2-soluble portion of a MeOH extract of stems of Capparis acutifolia using silica gel column chromatography (CC) and preparative TLC afforded compounds 1–6.
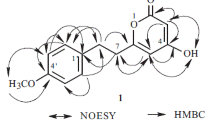
Compound 1 was isolated as a white powder. The HR-ESI-MS gave an [M + Na]+ ion at m/z 261.0737 (calcd for C12H14O5Na, 261.0739), consistent with a molecular formula of C12H14O5. IR absorption for the carbonyl (1715 cm–1) function was observed. The signals of the 1H and 13C NMR spectra of 1 were assigned to two aromatic protons, a methoxy, a methylenedioxy, and a propoxycarbonyl group. Comparison of the 1H NMR data of 1 with those of 5-methoxy-3,4-methylenedioxybenzoic acid [7] suggested that their structures were closely related, except for the propoxycarbonyl group [δ 1.02 (3H, t, J = 7.0 Hz, H-3′), 1.78 (2H, m, H-2′), 4.26 (2H, t, J = 7.0 Hz, H-1′)] at C-1 of 1, replacing the 1-COOH group of 5-methoxy-3,4-methylenedioxybenzoic acid [7]. This was supported by HMBC correlations between H-2 (δ 7.33), H-6 (δ 7.21), H-1′ (δ 4.26) and C=O (δ 166.4) and NOESY correlations between H-2 (δ 7.33) and H-1′ (δ 4.26). The full assignment of 1H and 13C NMR resonances was supported by 1H–1H COSY, DEPT, HSQC, NOESY, and HMBC (Fig. 1) spectral analyses. On the basis of the above data, the structure of 1 was elucidated as propyl 7-methoxybenzo[d][1,3]dioxole-5-carboxylate.
The known isolates were readily identified by a comparison of physical and spectroscopic data (UV, IR, 1H NMR, [α]D, and MS) with corresponding authentic samples or literature values, and they included rutin (2) [8], 3β-hydroxystigmast-5-en-7-one (3) [9], 6β-hydroxystigmast-4-en-3-one (4) [10], sitosterol trans-ferulate (5) [11], and sitosterol cis-ferulate (6) [12].
The anti-inflammatory effects of the isolated compounds from the stems of C. acutifolia were evaluated by suppressing fMet-Leu-Phe (fMLP)-induced O2 ·– generation by human neutrophils. Among the isolated compounds, rutin (2) exhibited potent inhibition, with IC50 value of 1.26 ± 0.15 μg/mL, against formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP)-induced superoxide anion (O2·–) generation.
Experimental
General Experimental Procedure. Ultraviolet (UV) spectra were obtained on a Jasco UV-240 spectrophotometer. Infrared (IR) spectra (neat or KBr) were recorded on a PerkinElmer 2000 FT-IR spectrometer. Nuclear magnetic resonance (NMR) spectra, including correlation spectroscopy (COSY), nuclear Overhauser effect spectrometry (NOESY), heteronuclear multiple-bond correlation (HMBC), and heteronuclear single-quantum coherence (HSQC) experiments, were recorded on a Varian Inova500 spectrometer operating at 500 MHz (1H) and 125 MHz (13C), with chemical shifts given in ppm (δ) using tetramethylsilane (TMS) as an internal standard. Electrospray ionization (ESI) and high-resolution electrospray ionization (HR-ESI)-mass spectra were recorded on a Bruker APEX II mass spectrometer. Silica gel (70–230, 230–400 mesh) (Merck) was used for column chromatography (CC). Silica gel 60 F-254 (Merck) was used for thin-layer chromatography (TLC) and preparative thin-layer chromatography (PTLC).
Plant Material. The stems of C. acutifolia were collected from Majia, Pingtung County, Taiwan, in April 2005, and a voucher specimen (CA200504) was deposited in the Department of Pharmacy, Tajen University, Pingtung, Taiwan.
Extraction and Separation of Compounds. The dried stems (1.7 kg) of C. acutifolia were pulverized and extracted three times with MeOH (15 L each) for 3 days. The methanol extract (145 g) was partitioned between CH2Cl2 and H2O (1:1) to afford CH2Cl2-soluble (fraction A, 64 g) and H2O-soluble (fraction B, 80 g) fractions. The CH2Cl2-soluble fraction (64 g) was chromatographed on silica gel (70–230 mesh, 2.9 kg), eluting with CH2Cl2, gradually increasing the polarity with MeOH to give 10 fractions (A1–A10). Fraction A3 (5.6 g) was separated by column chromatography on silica gel (230–400 mesh, 253g), eluting with CH2Cl2–MeOH (15:1–1:1) to yield nine fractions (A3-1–A3-9). Part (127 mg) of fraction A3-5 was purified by preparative TLC (silica gel, CHCl3–MeOH, 20:1) to afford propyl 7-methoxybenzo[d][1,3]dioxole-5-carboxylate (1) (4.7 mg). Fraction A4 (6.2 g) was separated by column chromatography on silica gel (230–400 mesh, 280 g), eluting with CH2Cl2–MeOH (8:1–0:1) to yield 10 fractions (A4-1–A4-10). Part (148 mg) of fraction A4-3 was purified by preparative TLC (silica gel, CHCl3–MeOH, 8:1) to afford sitosterol trans-ferulate (5) (6.6 mg). Part (152 mg) of fraction A4-4 was purified by preparative TLC (silica gel, CH2Cl2–MeOH, 7:1) to afford sitosterol cis-ferulate (6) (4.1 mg). Fraction A5 (5.4 g) was separated by column chromatography on silica gel (230–400 mesh, 245 g), eluting with CH2Cl2–MeOH (7:1–0:1) to yield 10 fractions (A5-1–A5-10). Part (175 mg) of fraction A5-4 was purified by preparative TLC (silica gel, CHCl3–MeOH, 6:1) to give 6β-hydroxystigmast-4-en-3-one (4) (5.5 mg). Part (164 mg) of fraction A5-5 was purified by preparative TLC (silica gel, CH2Cl2–MeOH, 5:1) to afford 3β-hydroxystigmast-5-en-7-one (3) (4.7 mg). Fraction A9 (4.9 g) was separated by column chromatography on silica gel (230–400 mesh, 225 g), eluting with CH2Cl2–MeOH (4:1–0:1) to yield eight fractions (A9-1–A9-8). Part (205 mg) of fraction A9-5 was purified by preparative TLC (silica gel, CHCl3–MeOH, 2:5) to obtain rutin (2) (6.1 mg).
Propyl 7-methoxybenzo[ d ][1,3]dioxole-5-carboxylate (1). White powder, mp 90–92°C (EtOAc). UV (MeOH, λmax, nm): 278 (3.96). IR (KBr, νmax, cm–1): 1715 (C=O). 1H NMR (500 MHz, CDCl3, δ, ppm, J/Hz): 1.02 (3H, t, J = 7.0, H-3′), 1.78 (2H, m, H-2′), 3.94 (3H, s, 3-OMe), 4.26 (2H, t, J = 7.0, H-1′), 6.06 (2H, s, OCH2O), 7.21 (1H, d, J = 1.5, H-6), 7.33 (1H, d, J = 1.5, H-2). 13C NMR (125 MHz, CDCl3, δ, ppm): 10.5 (C-3′), 22.1 (C-2′), 56.5 (3-OMe), 66.3 (C-1′), 102.3 (OCH2O), 103.9 (C-6), 109.9 (C-2), 124.5 (C-1), 139.4 (C-4), 143.3 (C-3), 148.6 (C-5), 166.4 (C=O). ESI-MS m/z 261 [M + Na]+. HR-ESI-MS m/z 261.0737 [M + Na]+ (calcd for C12H14O5Na, 261.0739).
Biological Assay. The effect of the isolated compounds on neutrophil pro-inflammatory response was evaluated by monitoring the inhibition of superoxide anion generation in fMLP-activated human neutrophils in a concentration-dependent manner.
Preparation of Human Neutrophils. Human neutrophils from the venous blood [13] of healthy adult volunteers (20–35 years old) were isolated using a standard method of dextran sedimentation prior to centrifugation in a Ficoll Hypaque gradient and hypotonic lysis of erythrocytes as previously described [14]. Purified neutrophils containing > 98% viable cells, as determined by the Trypan Blue exclusion method, were resuspended in HBSS buffer at pH 7.4 and were maintained at 4°C prior to use.
Measurement of O 2 ·– Generation. The assay for measurement of O2 ·– generation was based on the SOD-inhibitable reduction of ferricytochrome c [15]. In brief, neutrophils (1 × 106 cells/mL) pretreated with the various test agents at 37°C for 5 min were stimulated with fMLP (1 μmol/L) in the presence of ferricytochrome c (0.5 mg/mL). Extracellular O2·– production was assessed with a UV spectrophotometer at 550 nm (Hitachi U-3010, Tokyo, Japan). The percentage of superoxide inhibition of the test compound was calculated as the percentage of inhibition = {(control – resting) – (compound – resting)}/(control – resting) × 100. The software SigmaPlot was used for determining the IC50 values.
Statistical Analysis. Results are presented as average ± standard error of the mean (SEM) (n = 4), and comparisons were made using Student′s t-test. A probability of 0.05 or less was considered significant.
References
T. S. Liu and J. C. Liao, Capparaceae in Flora of Taiwan, Vol. 2, 2nd Ed.; Editorial Committee of the Flora of Taiwan, Taipei, Taiwan, 1996, pp. 734–744.
W. L. Li, L. Yu, and Y. B. Ji, Asian J. Chem., 26, 3435 (2014).
A. K. Ibrahim, A. I. Youssef, A. S. Arafa, and S. A. Ahmed, Nat. Prod. Res., 27, 2149 (2013).
A. Kanaujia, R. Duggar, S. T. Pannakal, S. S. Yadav, C. K. Katiyar, V. Bansal, S. Anand, S. Sujatha, and B. S. Lakshmi, Bioorg. Med. Chem., 18, 3940 (2010).
P. Bigoniya, C. S. Singh, and B. Shrivastava, Int. J. Pharm. Biol. Sci., 3, 139 (2013).
X. X. Lei, Y. L. Feng, S. L. Yang, L. Z. Xu, and Y. Q. Li, Chem. Nat. Compd., 51, 40 (2015).
A. F. Barrero, M. M. Herrador, and P. Arteaga, Phytochemistry, 37, 1351 (1994).
R. Bencheraiet, A. Kabouche, Z. Kabouche, and M. Jay, Chem. Nat. Compd., 47, 814 (2011).
Y. Qiang, X. X. Zhang, H. Liu, and Y. R. Xu, Chem. Nat. Compd., 49, 1143 (2014).
Q. Zeng, J. Ye, J. Ren, X. R. Cheng, J. J. Qin, H. Z. Jin, and W. D. Zhang, Chem. Nat. Compd., 49, 486 (2013).
J. K. Winkler-Moser, H. S. Hwang, E. L. Bakota, and D. A. Palmquist, Food Chem., 169, 92 (2014).
T. Akihisa, K. Yasukawa, M. Yamaura, M. Ukiya, Y. Kimura, N. Shimizu, and K. Arai, J. Agr. Food Chem., 48, 2313 (2000).
A. Boyum, Scand. J. Clin. Lab. Invest., 97, 77 (1968).
D. English and B. R. Andersen, J. Immunol. Methods, 5, 249 (1974).
B. M. Babior, R. S. Kipnes, and J. T. Curnutte, J. Clin. Invest., 52, 741 (1973).
Acknowledgment
This research was supported by a grant from the Ministry of Science and Technology, Taiwan (NSC 101-2320-B-127-001-MY3), awarded to Prof. J.-J. Chen.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2017, pp. 21–23.
Rights and permissions
About this article
Cite this article
Chen, JJ., Kuo, WL., Liao, HR. et al. A New Benzenoid and Anti-Inflammatory Constituent of Capparis acutifolia . Chem Nat Compd 53, 21–23 (2017). https://doi.org/10.1007/s10600-017-1901-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-1901-y