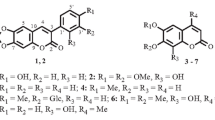
Two new coumarins, 6-hydroxy-7-methyl-3-(4′-methoxyphenyl)-coumarin (1) and 6-hydroxy-5-methoxy-7-methyl-3-(4′-methoxyphenyl)-coumarin (2), together with five known coumarins (3–7), were isolated from the leaves of Nicotiana tabacum. Their structures were determined by means of HR-ESI-MS and extensive 1D and 2D NMR spectroscopic studies. Compounds 1–7 were tested for their anti-tobacco mosaic virus (anti-TMV) activities. Compound 2 showed high anti-TMV activity with inhibition rate of 28.6%. This rate is close to that of the positive control. The other compounds also showed potential anti-TMV activity with inhibition rates in the ranges of 13.7–23.2%, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Nicotiana tabacum, tobacco, is a stout herbaceous plant in the Solanaceae (nightshade family) that originated in the tropical America (South America, Mexico, and the West Indies) and now cultivated worldwide as the primary commercial source of tobacco, which is smoked or chewed as a drug for its mild stimulant effects [1]. In addition, N. tabacum is also used as insecticides, anesthetics, diaphoretics, sedatives, and emetic agents in Chinese folk medicines because it contains many useful chemical compounds [1–3]. Previous phytochemical studies of tobacco have shown the presence of sesquiterpene [4], alkaloids [5, 6], lignans [7, 8], flavonoids [9, 10], phenylpropanoids [11], chromanones [12], biphenyls [13], phenolic amides [14], isocoumarins [15], and their homologs.
herefore, the multipurpose utilization of this plant is an interesting and topical and is attracting more and more attention. Our continuing phytochemical research on the tobacco leaves of Yunyan-85 (a variety of N. tabacum) led to the isolation of two new (1–2) and five known (3–7) coumarins. This paper deals with the isolation, structural elucidation, and anti-TMV activities of these compounds.
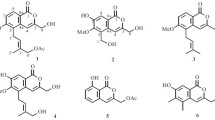
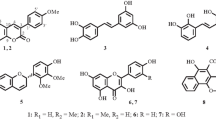
The 1H and 13C NMR data of compounds 1 and 2 are listed in Table 1. The known compounds, compared with literature data, were identied as muralatin G (3) [16], scopoletin (4) [17], scopoline (5) [17], fraxetin (6) [18], and (+)-decursinol (7) [19].

Compound 1 was isolated as a yellow gum. High-resolution ESI-MS analysis gave a quasi-molecular ion at m/z 305.0782 [M + Na]+ (calcd 305.0790), consistent with a molecular formula of C17H14O4, which indicated 11 degrees of unsaturation. Its UV spectrum showed maximum absorption at 280, 228, and 210 nm. Strong absorption bands accounting for hydroxy (3440 cm–1), carbonyl (1718 cm–1), and aromatic groups (1595, 1502, 1463 cm–1) could also be observed in its IR spectrum. The 1H and 13C NMR spectra of 1 (Table 1) displayed signals for all 17 carbons and 14 protons, including a 3,6,7-subsitituted-coumarin system (δC 162.9 s, 123.0 s, 140.2 d, 112.1 d, 152.4 s, 124.5 s, 119.0 d, 146.5 s, and 117.1 s; δH 7.97 s, 7.00 s, and 6.83 s) [20], a p-methoxy substituted phenyl moiety [δC 128.2 s, 130.4 d (2C), 115.7 d (2C), 161.4 s; δH 7.73 (2H, d, J = 8.6 Hz), 6.93 (2H, d, J = 8.6 Hz)] [21], one methyl group (δC 16.8 q; δH 2.33 s), and one phenolic hydroxy group (δH 10.98 br.s). The HMBC correlation (Fig. 1) between H-2′, 6′ (δH 7.73) and C-3 (δC 123.0) and H-4 (δH 7.97) and C-1′ (δC 128.2) indicated the linkage of the p-methoxy substituted phenyl moiety to be at the C-3 position. The location of the methyl group was assigned to the C-7 position on the basis of HMBC correlations between the methyl proton signals (δH 2.33 s) and C-6 (δC 152.4), C-7 (δC 124.5), and C-8 (δC 119.0). The phenolic hydroxy group located at C-6 was confirmed by the HMBC correlations of the phenolic hydroxy proton (δH 10.98) with C-5 (δC 112.1), C-6 (δC 152.4), and C-7 (δC 124.5). On the basis of the above evidence, the structure of 1 was established as 6-hydroxy-7-methyl-3-(4′-methoxyphenyl)-coumarin.
Compound 2 was also obtained as a yellow gum and showed a sodiated molecular ion at m/z 335.0899 [M + Na]+ in the HR-ESI-MS (calcd m/z 335.0895). The 1H and 13C NMR spectra data of 2 were similar to those of 1. The obvious chemical shift differences resulted from the substituent group variation on the benzene ring. The appearance of one methoxy group signal (δC 61.0 q, δH 3.87 s) and the disappearance of one aromatic proton signal were observed in compound 2. These changes indicated that, compared with compound 1, compound 2 should possess an additional methoxy group. Further analysis of the HMBC correlations suggested that the two methoxy groups are located at C-5 and C-4′, the phenolic hydroxy group is located at C-6, and the methyl group is located at C-7 for compound 2. Accordingly, compound 2 was assigned the structure 6-hydroxy-5-methoxy-7-methyl-3-(4′-methoxyphenyl)-coumarin.
Since certain of the coumarins exhibit potential anti-TMV activity [20, 21], compounds 1–7 were tested for their anti-tobacco mosaic virus activity. The anti-TMV activities were tested using the half-leaf method [22]. Ningnanmycin (a commercial product for treating plant disease in China), with inhibition rate of 32.2%, was used as a positive control. Their antiviral inhibition rates at the concentration of 20 μM are listed in Table 2. Compound 2 showed high anti-TMV activity with inhibition rate of 28.6%. This rate is close to that of the positive control. The other compounds also showed potential anti-TMV activity with inhibition rates in the ranges of 13.7–23.2%, respectively.
Experimental
General Methods. UV spectra were obtained using a Shimadzu UV-2401A spectrophotometer. IR spectra were obtained in KBr disc on a Bio-Rad Win inferred spectrophotometer. ESI-MS were measured on a VG Auto Spec-3000 MS spectrometer. 1H, 13C, and 2D NMR spectra were recorded on a Bruker DRX-500 instrument with TMS as internal standard. Column chromatography was performed on silica gel (200–300 mesh), or on silica gel H (10–40 μm, Qingdao Marine Chemical Inc., China). Preparative HPLC was carried out on an Agilent 1100 HPLC system equipped with a ZORBAX-C18 (21.2 mm × 250 mm, 7.0 μm) column and DAD detector.
Plant Material. The leaves of N. tabacum L. (tobacco leaves) were collected from Yuxi County, Yunnan Province, P. R. China, in September 2013. The tobacco variety is Yunyan-85, which is widely cultivated in China.
Extraction and Isolation. The air-dried and powdered tobacco leaves (5.2 kg) were extracted four times with 70% aqueous acetone (3 × 8 L) at room temperature and filtered. The solvent was evaporated in vacuo, and the crude extract (226 g) was subjected silica gel (200–300 mesh) column chromatography, eluting with a CHCl3–CH3OH gradient system (10:0, 9:1, 8:2, 7:3, 6:4, 5:5), to give six fractions A–F. Further separation of fraction C (8:2, 26.7 g) by silica gel column chromatography, eluting with CHCl3–(CH3)2CO (9:1–2:1), yielded mixtures C1–C6. Fraction C3 (7:3, 3.4 g) was subjected to silica gel column chromatography using petroleum ether–ethyl acetate and semipreparative HPLC (52% MeOH–H2O, flow rate 12 mL/min) to give 1 (12.2 mg), 2 (13.9 mg), 3 (8.2 mg), and 7 (6.3 mg). Fraction C4 (6:4, 2.8 g) was subjected to silica gel column chromatography using petroleum ether–ethyl acetate and semipreparative HPLC (48% MeOH–H2O, flow rate 12 mL/min) to give 4 (32.6 mg) and 6 (25.7 mg). Fraction C5 (1:1, 7.6 g) was subjected to silica gel column chromatography using petroleum ether–ethyl acetate and semipreparative HPLC (38% MeOH–H2O, flow rate 12 mL/min) to give 5 (41.8 mg).
6-Hydroxy-7-methyl-3-(4′-methoxyphenyl)-coumarin (1). C17H14O4, obtained as a yellow gum. UV (CH3OH, λmax, nm) (log ε): 280 (3.82), 228 (4.07), 210 (4.21). IR (KBr, v max, cm–1): 3440, 1718, 1595, 1502, 1463, 1418, 1272, 1160, 1057, 863, 784. 13C and 1H NMR data (125 MHz and 500 MHz), see Table1. Positive ESI-MS m/z 305 [M + Na]+; positive HR-ESI-MS m/z 305.0782 [M + Na]+ (calcd for C17H14NaO4, 305.0790).
6-Hydroxy-5-methoxy-7-methyl-3-(4′-methoxyphenyl)-coumarin (2). C18H16O5, obtained as a yellow gum. UV (CH3OH, λmax, nm) (log ε): 285 (3.98), 231 (4.22), 210 (4.43). IR (KBr, v max, cm–1): 3446, 1722, 1600, 1548, 1458, 1412, 1282, 1156, 1063, 874, 790. 13C and 1H NMR data (125 MHz and 500 MHz), see Table1. Positive ESI-MS m/z 335 [M + Na]+; positive HR-ESI-MS m/z 335.0899 [M + Na]+ (calcd for C18H16NaO5, 335.0895).
References
The Editorial Committee of the Administration Bureau of Flora of China, Flora of China, Vol. 67, Beijing Science and Technology Press, Beijing, 2005.
A. Rodgman and T. A. Perfetti, The Chemical Components of Tobacco and Tobacco Smoke. CRC Press, Taylor and Francis, Boca Raton, Florida, 2008.
M. M. Miao, L. Li, Q. P. Shen, C. B. Liu, Y. K. Li, T. Zhang, F. M. Zhang, P. He, K. M. Wang, R. Z. Zhu, Y. K. Chen, and G. Y. Yang, Fitoterapia, 103, 260 (2015).
G. Y. Yang, W. Zhao, Y. K. Chen, Z. Y. Chen, Q. F. Hu, and M. M. Miao, Asian. J. Chem., 25, 4932 (2013).
X. C. Wei, S. C. Sumithran, A. G. Deaciuc, H. R. Burton, L. P. Bush, L. P. Dwoskin, and P. A. Crooks, Life Sci., 78, 495 (2005).
T. Braumann, G. Nicolaus, W. Hahn, and H. Elmenhorst, Phytochemistry, 29, 3693 (1990).
Y. K. Chen, X. S. Li, G. Y. Yang, Z. Y. Chen, Q. F. Hu, and M. M. Miao, J. Asian. Nat. Prod. Res., 14, 450 (2012).
X. M. Gao, X. S. Li, X. Z. Yang, H. X. Mu, Y. K. Chen, G. Y. Yang, and Q. F. Hu, Heterocycles, 85, 147 (2012).
J. X. Chen, H. Q. Leng, Y. X. Duan, W. Zhao, G. Y. Yang, Y. D. Guo, Y. K. Chen, and Q. F. Hu, Phytochem. Lett., 6, 144 (2013).
W. Zhao, L. Li, P. Lei, L. Yang, S. Z. Shang, J. G. Tang, G. Y. Yang, Y. K. Chen, and M. M. Miao, Phytochem. Lett., 12, 125 (2015).
H. Q. Leng, J. X. Chen, Y. Hang, Y. X. Duan, G. Y. Yang, Y. K. Chen, Y. D. Guo, Q. F. Hu, and M. M. Miao, Chem. Nat. Compd., 49, 1028 (2014).
G. Y. Yang, W. Zhao, T. Zhang, Y. X. Duan, Z. H. Liu, M. M. Miao, and Y. K. Chen, Heterocycles, 89, 183 (2014).
S. Z. Shang, W. X. Xu, P. Lei, W. Zhao, J. G. Tang, M. M. Miao, H. D. Sun, J. X. Pu, Y. K. Chen, and G. Y. Yang, Fitoterapia, 99, 35 (2014).
S. Z. Shang, Y. X. Duan, X. Zhang, J. X. Pu, H. D. Sun, Z. Y. Chen, Mi. M. Miao, G. Y. Yang, and Y. K. Chen, Phytochem. Lett., 7, 413 (2014).
S. Z. Shang, W. X. Xu, L. Li, J. G. Tang, W. Zhao, P. Lei, M. M. Miao, H. D. Sun, J. X. Pu, Y. K. Chen, and G. Y. Yang, Phytochem. Lett., 11, 53 (2015).
H. N. Lv, S. Wang, K. W. Zeng, J. Li, X. Y. Guo, D. Ferreira, J. K. Zjawiony, P. F. Tu, and Y. Jiang, J. Nat. Prod., 78, 279 (2015).
D. Q. Yu and J. S. Yang, Handbook of Analytical Chemistry (7th Volume), Nuclear Magnetic Resonance Spectroscopy, Chemical Industry Press, 2nd Ed., Beijing, 1999.
A. Hitoshti, K. Ichiro, I. Kazuhiko, and I. Kazuo, J. Nat. Prod., 49, 366 (1986).
J. H. Lee, H. B. Bang, S. Y. Han, and J. G. Jun, Tetrahedron Lett., 48, 2889 (2007).
C. Lei, W. X. Xu, J. Wu, S. J. Wang, J. Q. Sun, Z. Y. Chen, and G. Y. Yang, Chem. Nat. Compd., 51, 43 (2015).
Y. K. Li, B. Zhou, X. X. Wu, G. Du, Y. Q. Ye, X. M. Gao, and Q. F. Hu, Chem. Nat. Compd., 51, 50 (2015).
Q. F. Hu, B. Zhou, J. M. Huang, X. M. Gao, L. D. Shu, G. Y. Yang, and C. T. Che, J. Nat. Prod., 76, 292 (2013).
Acknowledgment
This research was supported by the National Natural Science Foundation of China (No. 31360081), the Basic Research Foundation of Yunnan Province (2013FB097), and the Basic Research Foundation of Yunnan Tobacco Industry Co. Ltd (2012JC01).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2016, pp. 852–854.
Rights and permissions
About this article
Cite this article
Liu, CB., Shen, QP., Wang, Y. et al. Coumarins from the Leaves of Nicotiana tabacum and Their Anti-Tobacco Mosaic Virus Activities. Chem Nat Compd 52, 992–995 (2016). https://doi.org/10.1007/s10600-016-1844-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-016-1844-8