Conjugates of 24-epibrassinolide and 24-epicastasterone with salicylic acid were prepared by reacting the brassinosteroids with 2-O-benzylsalicylic acid followed by hydrogenolysis of the O-benzyl groups. The synthesized conjugates increased the survival of millet sprouts under heat and salt stress and decreased the accumulation in them of lipid peroxidation products. The action of the synthesized conjugates exceeded markedly the effects of the corresponding brassinosteroids, salicylic acid, and mixtures of these phytohormones.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Brassinosteroids (BR) are phytohormones with extremely high physiological activity, including stress-protective [1, 2]. Much evidence of the involvement of the plant hormonal system in carrying out the growth-stimulating and stress-protective effects of BR has accumulated over the last decades. Synergism between BR and gibberellins and BR and auxins was noted during growth stimulation [2]. Experimental results were also obtained regarding interruption of BR and salicylic acid (SA) signaling pathways because protein NPR1 was activated in signal transduction of both phytohormones [3]. However, SA is known to induce plant stability to stressors also through pathways not related to NPR1 [4].
Reports of the functional interaction of BR with other phytohormones prompted the synthesis of BR conjugates with various physiologically active compounds [5, 6].
The goal of the present work was to synthesize BR conjugates with SA and to assess their stress-protective action on young Panicum miliaceum L.
Attempts to prepare steroidal salicylates using SA anhydride or chloride found that the acylation was non-specific and formed a complicated product mixture. A study of the reaction of BR with an excess of acetyl-SA anhydride, which was prepared in situ from the acid through the action of dicyclohexylcarbodiimide (DCC) in dioxane, showed that 3,22,23-triacetoxy-2-(2′-acetoxybenzyloxy)-derivatives were formed, i.e., acetylation of the hydroxyls on C-3, C-22, and C-23 occurred in addition to acylation of the 2-position by acetyl-SA. All esters were saponified under the hydrolysis conditions so that monoesters of acetyl-SA could not be obtained.

2-O-Benzylsalicylic acid, which was prepared from SA by refluxing in Me2CO with an excess of benzylbromide in the presence of K2CO3 followed by saponification of the resulting ester [7], was used as a reagent. 2-Acyl-derivatives 4 and 5 were synthesized by reacting 24-epibrassinolide (1, 24-EBL) and 24-epicastasterone (2, 24-ECS), respectively, with 2-O-benzylsalicylic acid anhydride (3), which was prepared in situ from the acid. The reaction occurred at room temperature with a slight excess of anhydride 3.
The structures of 4 and 5 were confirmed by a significant downfield shift in the PMR spectrum of the H-2 resonance (resonances of H-3, H-22, and H-23 were practically constant) and the presence of a 2H singlet for benzyl methylene protons (δ 5.14 ppm) and resonances of nine aromatic benzene protons. The last in 4 had chemical shifts δ 7.01–7.06 (2H), 7.34–7.49 (6H), and 7.81 ppm; in 5, 6.99–7.04 (2H), 7.32–7.47 (6H), and 7.80 ppm. A peak for the molecular ion in mass spectra of the compounds, the presence of three UV absorption bands (λ 211, 234sh, 293 nm), and the appearance of an additional peak for carbonyl stretching vibrations in the IR spectrum confirmed that the structures were correct.
The benzyl protection in 4 and 5 was removed by hydrogenolysis in MeOH over a Pd catalyst. This produced target products 6 and 7. Their PMR spectra showed a singlet for the OH protons in SA (δ 10.67 ppm). The number of aromatic-proton resonances decreased to four. Strong peaks for the molecular ions in mass spectra of the products and peaks for carbonyl stretching vibrations at 1677 and 1716 cm–1 for 6 and 1672 and 1702 cm–1 for 7 in the IR spectra confirmed that the structures were correct. UV spectra contained bands corresponding to SA absorption (λ 213, 239, 306 nm).
The physiological activity of SA-modified BR was assessed by comparing the effects of 24-EBL and 24-ECS and their SA conjugates on the heat and salt stability of young millet plants. We demonstrated earlier [8, 9] that the experimental plants were sensitive to the action of BR, which at concentrations of 10–8–10–6 M increased the heat stability of the sprouts. The effect of BR was greatest at a concentration of 10–7 M (Table 1). The positive effects of the conjugates of 24-EBL and 24-ECS with SA at concentrations of 10–8 and 10–7 M were greater than those of 24-EBL and 24-ECS at the corresponding concentrations. The positive effects of BR and their conjugates decreased at a concentration of 10–6 M although the differences between the compounds disappeared. Mixtures of BR and SA at concentrations of 10–8 and 10–7 M did not enhance the heat stability of the sprouts whereas higher concentrations decreased the survival of sprouts after heating.
Thus, the effects of conjugates of BR and SA differed substantially from those of a mixture of BR and SA. These differences may have been related to gradual release of free BR and SA from the conjugates whereas the two phytohormones were antagonistic if administered simultaneously. Such an effect could be related to common signaling mediators involved in signal transduction of these phytohormones into the cellular genetic apparatus. As already noted, NPR1 is implicated in signal transduction of both SA and BR [3]. Reactive oxygen species may also be common mediators involved in signal transduction of both BR and SA [4, 10, 11].
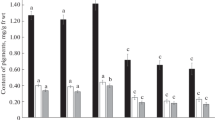
The effects of the BR conjugates with SA on the manifestation of lipid peroxidation (LPO) in millet sprouts were investigated in further experiments. Various types of stress can cause LPO in plants [12]. The contents of the LPO product malondialdehyde (MDA) in sprouts under physiologically normal conditions decreased under the influence of all indicated compounds and especially markedly after harmful heating. This indicated that oxidative damage of the sprouts decreased under the influence of BR (Fig. 1). The protective antioxidant action of the 24-EBL and 24-ECS conjugates with SA was more noticeable than the corresponding effects of BR and SA.
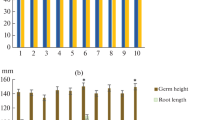
The effects of BR and their SA conjugates on the stability of young millet plants to salt stress were compared in the next series of experiments. Treatment of millet with BR modified by SA had a more substantial protective effect than the corresponding ordinary BR and SA (Fig. 2).
Effects of 1, 2, SA, and conjugates 6 and 7 on salt stability of millet sprouts after treatment with NaCl solution (500 mM) for 7 h (designations the same as in Fig. 1).
The 24-EBL and 24-ECS conjugates were also more effective under salt stress than starting BR and SA at preventing oxidative damage to the millet plants. This manifested as a lower content of the LPO product MDA in plants treated with BR modified with SA than in plants treated with ordinary BR and SA both with and without salt stress (Fig. 3).
Effects of 1, 2, SA, and conjugates 6 and 7 on MDA content (nmol/g of crude compound) in millet sprouts after incubation for 24 h (a) and after salt stress for 7 h (b) (designations the same as in Fig. 1).
The combination of exogenic stress phytohormones BR and SA as conjugates may have initiated a broader spectrum of protective reactions than BR and SA separately.
The role of SA in plant stability to abiotic stresses was associated with normalization of the photosynthetic apparatus [13] and a change of hormonal balance, which appeared as an increase in the contents of abscisic and indoleacetic acids [14]. The activity of antioxidant enzymes was increased through the action of SA in many works [15–18].
Preliminary treatment of cucumber sprouts with SA elevated expression of an alternative oxidase gene and thereby decreased oxidative damage by low temperatures [19].
As already noted, NPR1 protein was implicated in transduction of BR and SA signals. However, it remains unclear how the signaling pathways associated with NPR1 and ROS interact. It was recently reported that NPR1 formed monomers (was reduced) after an oxidative explosion caused by SA and the subsequent increase in the reductant concentration [20]. Thus, ROS and NPR1 were probably components of a single SA-signaling network and, possibly, BR signaling. Stress could increase the SA and BR contents in cells. In turn, this induced ROS generation and reductant (in particular, thioredoxin that can reduce NPR1) accumulation in response [21].
More detailed research on the phenomenology of the influence of SA-modified BR on actual stress-protection plant systems is required for a deeper understanding of the stress-protection action of BR. In addition, special research is needed in order to identify the components of signals induced by these compounds that are transduced into the genetic apparatus of plants.
Experimental
Melting points were determined on a Kofler apparatus. UV spectra were recorded in MeOH on a Specord UV/Vis instrument. IR spectra were obtained from KBr pellets or films on a UR-20 instrument. PMR and 13C NMR spectra were recorded in CDCl3 using residual solvent peaks as internal standards (δH 7.26 and δC 77.16 ppm) on a Bruker Avance DRX-500 instrument (operating frequency 500 MHz for 1H and 125 MHz for 13C). Positive-ion mass spectra were obtained in an LCQ Fleet mass spectrometer (Thermo Electron Corp.) using chemical ionization at atmospheric pressure (APCI). High-resolution mass spectra were taken using electrospray ionization on an LTQ Orbitrap Velos instrument (Thermo Fisher Scientific). The course of reactions was monitored by TLC on Kieselgel 60 F254 plates with detection by anisaldehyde followed by heating or under a UV lamp. Reaction mixtures were separated by chromatography over silica gel 40/60 (Kieselgel 60, Merck).
(22 R ,23 R )-2- α(2′-Benzyloxybenzoyloxy)-3 α ,22,23-trihydroxy- β -homo-7-oxa-5-ergostan-6-one (4). A mixture of 2-(benzyloxy)benzoic acid (100 mg, 0.44 mmol) [7] and DCC (45 mg, 0.22 mmol) in anhydrous dioxane (2 mL) was stirred at room temperature for 1 h. The precipitate was filtered off. The filtrate and dimethylaminopyridine (2.4 mg, 0.02 mmol) were added to 24-EBL (1, 96mg, 0.2 mmol). The resulting solution was stirred at room temperature for 24 h. The solvent was evaporated. The solid was chromatographed over silica gel (eluent petroleum ether–EtOAc, 10:1) to afford 4 (82 mg, 59%) as an oil. UV spectrum (MeOH, λmax, nm) (ε): 211 (17600), 234 (sh), 293 (3000). IR spectrum (film, ν, cm–1): 3450, 2960, 2875, 1722, 1720, 1460, 1305, 1245. 1H NMR spectrum (CDCl3, δ, ppm, J/Hz): 0.70 (3H, s, Me-18), 0.85 (3H, d, J = 7.0, Me-28), 0.87 (3H, d, J = 7.0, Me-27), 0.92 (3H, d, J = 7.0, Me-26), 0.96 (3H, d, J = 7.0, Me-21), 0.98 (3H, s, Me-19), 3.10 (1H, dd, J = 12.0, 4.0, H-5), 3.41 (1H, m, H-23), 3.68 (1H, m, H-22), 4.01–4.11 (3H, m, H-3, 7), 5.05 (1H, m, H-2), 5.14 (2H, s, OCH 2 Ph), 7.01–7.06 (2H, m, H-3′, 5′), 7.34–7.49 (6H, m, H-4′, OCH2 Ph), 7.81 (1H, dd, J = 7.0, 2.0, H-6′). 13C NMR spectrum (CDCl3, δ, ppm): 10.98 (q), 11.76 (q), 12.50 (q), 15.44 (q), 17.44 (q), 22.26 (q), 22.34 (t), 24.88 (t), 27.14 (d), 27.80 (t), 30.59 (t), 37.75 (t), 38.53 (s), 39.32 (d), 39.70 (t), 40.28 (d), 41.08 (d), 41.51 (d), 42.57 (s), 51.38 (d), 52.72 (d), 58.27 (d), 66.28 (d), 70.53 (t), 71.08 (t), 72.49 (d), 72.69 (d), 76.50 (d), 113.55 (d), 120.75 (s), 120.90 (d), 127.82 (2d), 128.57 (d), 128.92 (2d), 132.04 (d), 133.82 (d), 136.22 (s), 158.01 (s), 166.11 (s), 176.06 (s). Mass spectrum, m/z (I rel, %): 691 [M + H]+ (100). Found: m/z 690.4132 [M]+. C42H58O8. Calcd: MM 690.4132.
(22 R ,23 R )-2 α -(2′-Benzyloxybenzoyloxy)-3 α ,22,23-trihydroxy-5 α -ergostan-6-one (5) was synthesized by the method described for 4 from 24-ECS (2, 115 mg, 0.25 mmol) to afford 5 (73 mg, 54%) as an oil. UV spectrum (MeOH, λmax, nm) (ε): 211 (18000), 234 (sh), 293 (3600). IR spectrum (film, ν, cm–1): 3450, 2950, 2865, 1705, 1703, 1452, 1308, 1261. 1H NMR spectrum (CDCl3, δ, ppm, J/Hz): 0.66 (3H, s, Me-18), 0.81 (3H, s, Me-19), 0.84 (3H, d, J = 7.0, Me-28), 0.86 (3H, d, J = 7.0, Me-27), 0.91 (3H, d, J = 7.0, Me-26), 0.97 (3H, d, J = 7.0, Me-21), 2.68 (1H, dd, J = 12.0, 3.0, H-5), 3.41 (1H, m, H-23), 3.70 (1H, m, H-22), 4.08 (1H, m, H-3), 5.11 (1H, m, H-2), 5.14 (2H, s, ArOCH 2 Ph), 6.99–7.04 (2H, m, H-3′, 5′), 7.32–7.47 (6H, m, H-4′, OCH2 Ph), 7.80 (1H, dd, J = 8.0, 2.0, H-6′). 13C NMR spectrum (CDCl3, δ, ppm): 11.00 (q), 11.98 (q), 12.57 (q), 13.59 (q), 17.47 (q), 21.33 (t), 22.30 (q), 24.00 (t), 25.90 (t), 27.10 (d), 27.85 (t), 36.52 (t), 37.77 (d), 39.51 (t), 40.19 (d), 41.44 (d), 42.69 (s), 42.91 (s), 46.75 (t), 50.79 (d), 52.71 (d), 53.76 (d), 56.64 (d), 66.53 (d), 71.09 (t), 72.72 (d), 72.86 (d), 76.52 (d), 113.61 (d), 120.82 (s), 120.89 (d), 127.80 (2d), 128.50 (d), 128.88 (2d), 132.01 (d), 133.78 (d), 136.19 (s), 157.98 (s), 166.11 (s), 211.89 (s). Mass spectrum, m/z (I rel, %): 675 [M + H]+ (100), 639 (24), 447 (58), 429 (74), 411 (81). Found: m/z 674.4184 [M]+. C42H58O7. Calcd: MM 674.4183.
(22 R ,23 R )-2 α -(2′-Hydroxybenzoyloxy)-3 α ,22,23-trihydroxy- β -homo-7-oxa-5 α -ergostan-6-one (6). A solution of 4 (79 mg, 0.114 mmol) in MeOH (2 mL) was treated with Pd/C (5%) as a catalyst (24 mg) and stirred under a stream of H2 456 for 1 h. The catalyst was filtered off through a layer of silica gel. The solvent was evaporated to afford 6 (67 mg, 94%), mp 203–205°C (EtOAc–hexane). UV spectrum (MeOH, λmax, nm) (ε): 213 (17600), 239 (6400), 306 (3400). IR spectrum (KBr, ν, cm–1): 3510, 2958, 2930, 2860, 1716, 1677, 1302, 1210. 1H NMR spectrum (CDCl3, δ, ppm, J/Hz): 0.70 (3H, s, Me-18), 0.83 (3H, d, J = 7.0, Me-28), 0.86 (3H, d, J = 7.0, Me-27), 0.90 (3H, d, J = 7.0, Me-26), 0.93 (3H, d, J = 7.0, Me-21), 0.99 (3H, s, Me-19), 3.22 (1H, dd, J = 12.0, 4.0, H-5), 3.41 (1H, m, H-23), 3.69 (1H, m, H-22), 4.09 (2H, m, H-7), 4.25 (1H, m, H-3), 5.08 (1H, m, H-2), 6.87 (1H, m, H-5′), 6.98 (1H, d, J = 8.0, H-3′), 7.46 (1H, m, H-4′), 7.84 (1H, d, J = 7.0, H-6′), 10.67 (1H, s, ArOH). 13C NMR spectrum (CDCl3, δ, ppm): 11.00 (q), 11.77 (q), 12.46 (q), 15.50 (q), 17.49 (q), 22.31 (q), 22.41 (t), 24.87 (t), 27.09 (d), 27.76 (t), 31.23 (t), 37.85 (t), 38.67 (s), 39.31 (d), 39.63 (t), 40.10 (d), 41.14 (d), 41.35 (d), 42.58 (s), 51.33 (d), 52.64 (d), 58.27 (d), 66.24 (d), 70.62 (t), 72.76 (d), 73.14 (d), 76.54 (d), 112.43 (s), 117.87 (d), 119.35 (d), 129.94 (d), 136.14 (d), 161.88 (s), 169.38 (s), 176.08 (s). Mass spectrum, m/z (I rel, %): 601 [M + H]+ (100), 583 [M + H – H2O]+ (42), 565 (47), 463 (16), 445 (38), 427 (15), 151 (72), 137 (61), 109 (23). Found: m/z 600.3665 [M]+. C35H52O8. Calcd: MM 600.3662.
(22 R ,23 R )-2 α -(2′-Hydroxybenzoyloxy)-3 α ,22,23-trihydroxy-5 α -ergostan-6-one (7) was synthesized by the method used for 6 from 5 (57 mg, 0.085 mmol) to afford 7 (49 mg, 98%), mp 207–209°C (EtOAc–hexane). UV spectrum (MeOH, λmax, nm) (ε): 213 (16000), 235 (5500), 306 (4000). IR spectrum (KBr, ν, cm–1): 3480, 2957, 2868, 1702, 1672, 1303, 1250. 1H NMR spectrum (CDCl3, δ, ppm, J/Hz): 0.66 (3H, s, Me-18), 0.82–0.87 (9H, m, Me-19, 27, 28), 0.89 (3H, d, J = 7.0, Me-26), 0.94 (3H, d, J = 7.0, Me-21), 2.80 (1H, dd, J = 12.0, 3.0, H-5), 3.46 (1H, m, H-23), 3.74 (1H, m, H-22), 4.26 (1H, m, H-3), 5.16 (1H, m, H-2), 6.87 (1H, m, H-5′), 6.97 (1H, d, J = 8.0, H-3′), 7.45 (1H, m, H-4′), 7.87 (1H, d, J = 8.0, H-6′), 10.67 (1H, s, ArOH). 13C NMR spectrum (CDCl3, δ, ppm): 11.08 (q), 11.99 (q), 12.52 (q), 13.65 (q), 17.63 (q), 21.42 (t), 22.45 (q), 23.99 (t), 26.59 (t), 27.01 (d), 27.80 (t), 36.64 (t), 37.76 (d), 39.45 (t), 39.66 (d), 40.98 (d), 42.74 (s), 42.92 (s), 46.62 (t), 50.81 (d), 52.61 (d), 53.85 (d), 56.58 (d), 73.38 (d), 66.57 (d), 73.06 (d), 76.78 (d), 112.48 (s), 117.83 (d), 119.37 (d), 130.04 (d), 136.11 (d), 161.83 (s), 169.35 (s), 211.91 (s). Mass spectrum, m/z (I rel, %): 583 [M – H]– (100). Found: m/z 584.3710 [M]+. C35H52O7. Calcd: MM 584.3713.
Influence of BR and Their SA Conjugates on Heat and Salt Stability of the Plants. The action of BR on the heat stability of the plants was studied using etiolated millet sprouts (Konstantinovskoe variety) [8]. Four-day sprouts were stored for 24 h in solutions of the tested BR and their SA conjugates in addition to SA and a mixture of BR with SA. Control sprouts were incubated in purified tap water and exposed to potentially lethal heat in a water ultrathermostat (47°C, 10 min). The content of LPO product MDA in the sprouts was determined 1 d after heating. The relative amount of surviving sprouts was counted on the fourth day.
The influence of SA-modified BR on salt stability was studied on 9–10-day plants grown in Hoagland nutrient solution (0.2 N). The test preparations were added to the medium 1 d before salt stress. The optimum BR concentrations that increased the plant salt stability were established earlier [9]. Salt stress was created by adding NaCl to a final concentration of 500 mM and exposing for 7 h [9]. The MDA content in leaves was determined immediately after salt stress. The number of surviving plants was counted on the fourth day.
The contents of LPO products (calculated as MDA) were determined from the reaction with thiobarbituric acid [22].
The experiments were performed in triplicate with 3–4 repetitions in each series. Each repetition consisted of at least 30 plants for the survival assessment. Biochemical analyses were repeated with an average sample from 6–7 plants. Figures 1–3 show the arithmetic averages and their mean-square errors (M ± m).
References
V. Khripach, V. Zhabinskii, and A. De Groot, Ann. Bot., 86, 441 (2000).
A. Bajguz and S. Hayat, Plant Physiol. Biochem., 47, 1 (2009).
U. K. Divi, T. Rahman, and P. Krishna, BMC Plant Biol., 10, 151 (2010).
Yu. E. Kolupaev and T. O. Yastreb, Fiziol. Biokhim. Kul′t. Rast., 45, 113 (2013).
R. P. Litvinovskaya, P. S. Minin, M. E. Raiman, G. A. Zhilitskaya, A. L. Kurtikova, K. G. Kozharnovich, M. V. Derevyanchuk, V. S. Kravets, and V. A. Khripach, Chem. Nat. Compd., 49, 478 (2013).
RB Pat. No. 14986, Oct. 30, 2009; Byull. Izobret., No. 3 (2011).
M. M. Johnson, J. M. Naidoo, M. A. Fernandes, E. M. Mmutlane, W. A. L. van Otterlo, and C. B. de Koning, J. Org. Chem., 75, 8701 (2010).
A. A. Vayner, Yu. E. Kolupaev, N. V. Shvidenko, and V. A. Khripach, Biotechnol. Acta, 7 (5), 77 (2014).
A. A. Vainer, Yu. E. Kolupaev, and V. A. Khripach, Fiziol. Rast. Genet., 46, 428 (2014).
X.-J. Xia, Y.-J. Wang, and Y.-H. Zhou, Plant Physiol., 150, 801 (2009).
Yu. E. Kolupaev, A. A. Vayner, T. O. Yastreb, A. I. Oboznyi, and V. A. Khripach, Appl. Biochem. Microbiol., 50, 593 (2014).
K. Apel and H. Hirt, Annu. Rev. Plant Biol., 55, 373 (2004).
A. Krantev, R. Yordanova, T. Janda, G. Szalai, and L. Popova, J. Plant Physiol., 165, 920 (2008).
D. Sircar and A. Mitra, J. Plant Physiol., 166, 1370 (2009).
L. P. Popova, L. T. Maslenkova, R. Y. Yordanova, A. P. Ivanova, A. P. Krantev, G. Szalai, and T. Janda, Plant Physiol. Biochem., 47, 224 (2009).
H. Wang, T. Feng, X. Peng, M. Yan, and X. Tang, Ecotoxicol. Environ. Saf., 72, 1354 (2009).
A. Kadioglu, N. Saruhan, A. Saglam, R. Terzi, and T. Acet, Plant Growth Regul., 64, 27 (2010).
M. G. Javid, A. Sorooshzadeh, F. Moradi, S. A. Sanavy, and I. Allahdadi, Aust. J. Crop Sci., 5, 726 (2011).
T. Lei, H. Feng, X. Sun. Q. L. Dai, F. Zhang, H. G. Liang, and H. H. Lin, Plant Growth Regul., 60, 35 (2010).
S. Peleg-Grossman, N. Melamed-Book, and A. Levine, Plant Signaling Behav., 7, 409 (2012).
C. An and Z. Mou, J. Integr. Plant Biol., 53, 412 (2011).
M. N. Merzlyak, S. I. Pogosyan, S. G. Yuferova, and V. A. Shevyreva, Biol. Nauki, No. 9, 86 (1978).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2016, pp. 394–398.
Rights and permissions
About this article
Cite this article
Litvinovskaya, R.P., Vayner, A.A., Zhylitskaya, H.A. et al. Synthesis and Stress-Protective Action on Plants of Brassinosteroid Conjugates with Salicylic Acid. Chem Nat Compd 52, 452–457 (2016). https://doi.org/10.1007/s10600-016-1671-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-016-1671-y