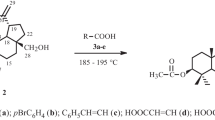
Betulinic acid was sulfonated by sulfamic acid in the presence of urea in homogeneous 1,4-dioxane or DMF solution at 65–75°C in 2.5–3.5 h to give betulinic acid 3-sulfate, the structure of which was confirmed by IR and 13C NMR spectroscopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Betulinic acid (BA) (1) exhibits the most pronounced antitumor activity of lupane triterpenoids found in the bark and leaves of many plants. However, isolation of BA from plant raw material is economically unfeasible because of its low content (up to 0.1%) [1]. Bark of Betula pendula Roth. (European white birch) contains up to 35% of betulin, a biosynthetic precursor of BA [2]. It was shown that the antiproliferative activity of BA is an order of magnitude greater than that of the known antineoplastic agent doxorubicin against human melanoma cells [3] and inhibits the growth of cancer cells [4]. A study of the biological activity of BA 3-sulfate showed that it was a stronger complement inhibitor than the currently used medicines [5].
In many instances, the efficacy of medicines depends on their water-solubility.
The broad spectrum of biological activity of BA and its sulfate prompted the development of effective BA sulfonation methods [5–8].
Traditional methods for synthesizing triterpenoid sulfates are based on the use of H2SO4, SO3, and chlorosulfonic acid (ClSO3H) [6–8]. Sulfamic acid (NH2SO3H), in contrast with the aforementioned aggressive reagents, is a stable, nonhygroscopic, crystalline compound that is soluble in DMF and 1,4-dioxane and is just as acidic as H2SO4 [8]. Sulfamic acid is produced industrially by the reaction of urea and conc. H2SO4. Its reactivity is similar to that of SO3 complexes with tertiary amines (NR3·SO3) [8].
Previous research showed that sulfonation of betulin by NH2SO3H in the presence of urea in DMF and 1,4-dioxane formed betulin 3,28-disulfate in 97% yield. The double bond in the isopropenyl group of betulin was unaffected during the reaction with NH2SO3H [9]. The catalytic effect of urea during NH2SO3H sulfonation was explained by the formation of a donor–acceptor complex that was more reactive toward sulfonation [9, 10]. Sulfonation of BA by NH2SO3H has not been reported.
The goal of the present work was to study the reaction of BA and NH2SO3H in DMF and 1,4-dioxane in the presence of urea.

Sulfonation of BA by NH2SO3H in the presence of urea in DMF or 1,4-dioxane formed the ammonium salt of BA 3-sulfate (2), which was treated with H2SO4 (10%) to give BA 3-sulfonic acid (3a); with KOH or NaOH solution (3–5%), the stable salts 3b or 3c.
IR spectra of 3b and 3c, in contrast to that of BA, showed an absorption band at 830 cm–1 (SO), and a strong band at 1221 (SO2), which confirmed that 3b and 3c contained sulfate groups [11] in addition to 2 and 3a.
13C NMR spectra of the natural 3α-and 3β-stereomers of BA and 3α-and 3β-stereomers of BA sulfates isolated from Schefflera octophylla leaves were reported [11] and showed that the C-3 resonance in 3α-BA was observed at 75.5 ppm; in 3β-BA, at 78.2 ppm. The C-3 resonance of BA 3α-sulfate had a chemical shift of 82.9 ppm; of BA 3β-sulfate, 86.0 ppm.
A comparison of the 13C NMR spectra of starting BA and sulfates 3b and 3c showed that replacing the OH by SO3H shifted the C-3 resonance from 79.0 to 86.5 ppm.
Thus, it was established that reaction of BA and NH2SO3H in the presence of urea in DMF and 1,4-dioxane occurred in 2.5–3.5 h at 65–75°C. The yield of 2 was 96%; 3a, 94; 3b, 93; 3c, 95.
The advantage of BA sulfonation by NH2SO3H is the use of more available and less aggressive reagents. The nature of the solvent (DMF and 1,4-dioxane) did not substantially affect the course of BA sulfonation.
Experimental
General. IR spectra were taken from KBr pellets (3 mg of sample/300 mg of KBr) on a Tensor 27 FTIR spectrometer (Bruker, Germany) in the range 400–4000 cm–1. 13C NMR spectra were recorded in CD3OD with TMS = 0 on a Bruker Avance III spectrometer (600 MHz). Elemental analysis with simultaneous determination (%) of C, H, N, S, and O was performed on a FlashEATM 1112 analyzer (Thermo Quest, Italy). The K and Na contents were determined on an AAnalyst-400 atomic absorption spectrometer (PerkinElmer) using an acetylene/nitrous-oxide flame. Melting points were measured on an Electrothermal A9100 apparatus.
Betulinic acid (1) was prepared from betulin by the known method in two steps. The first consisted of betulin oxidation by CrO3 in AcOH to betulonic acid (61% yield, mp 247–249°C) [1]. The second involved betulonic acid reduction by NaBH4 in THF to betulinic acid (94% yield, mp 290–292°C) [1, 12, 13]. The BA used for sulfonation was the single β-stereomer. This was confirmed by the full agreement of its physicochemical properties with those published [7, 11–13]. DMF was dried by shaking with KOH and distilled over CaO. 1,4-Dioxane was dried over KOH and distilled over Na [14]. Salts 3b and 3c were purified by recrystallization from MeOH (75%).
Sulfonation of BA in DMF and 1,4-Dioxane. Ammonium 3 β - O -Sulfate-lup-20(29)-en-28-oate (2). DMF or 1,4-dioxane (50 mL) in a 100-mL three-necked flask equipped with a stirrer and thermometer was stirred vigorously; treated with NH2SO3H (1.46 g, 0.015 mol), urea (0.90 g), and 1 (4.56 g, 0.01 mol); heated on a water bath at 65–75°C for 2.5–3.5 h, cooled, diluted with H2O (100 mL), transferred to a separatory funnel, and extracted with BuOH (130–150 mL). The BuOH extract was washed with H2O and evaporated to dryness in vacuo. Yield of 2, 96%, mp (dec.) 147–150°C. C30H54N2O6S.
3 β - O -Sulfate-lup-20(29)-en-28-oic Acid (3a). The BuOH extract containing 2 was acidified with H2SO4 solution (10%) to pH 2–3. The BuOH layer was separated and concentrated in vacuo to afford 3a in 94% yield, mp (dec.) 139–143°C, lit. mp 140–142°C [14]. C30H48O6S.
Potassium 3 β - O -Sulfate-lup-20(29)-en-28-oate (3b). The BuOH extract containing 2 was worked up with KOH solution (3–4%) to pH 8–9. The BuOH layer was separated and concentrated in vacuo to afford 3b in 93% yield; 74% after recrystallization, mp (dec.) 312–315°C. C30H46O6SK2. IR spectrum (KBr, ν, cm–1): 838 (C–O–S), 1221 (O=S=O). 13C NMR spectrum (CD3OD, δ, ppm): 38.5 (C-1), 24.1 (C-2), 86.4 (C-3), 38.6 (C-4), 56.1 (C-5), 18.1 (C-6), 34.7 (C-7), 40.1 (C-8), 51.1 (C-9), 37.2 (C-10), 20.9 (C-11), 26.5 (C-12), 38.5 (C-13), 42.4 (C-14), 31.4 (C-15), 32.0 (C-16), 56.5 (C-17), 47.1 (C-18), 48.2 (C-19), 151.1 (C-20), 31.3 (C-21), 37.6 (C-22), 28.4 (C-23), 16.6 (C-24), 16.2 (C-25), 16.1 (C-26), 14.7 (C-27), 179.0 (C-28), 20.1 (C-29), 109.3 (C-30).
Sodium 3 β - O -Sulfate-lup-20(29)-en-28-oate (3c) was prepared analogously to 3b using NaOH solution (3–4%) in 95% yield, 76% after recrystallization, mp (dec.) 314–317°C. C30H46O6SNa2. The IR and 13C NMR spectra of 3c were identical to those published [15].
References
L. B. Son, A. P. Kaplun, A. A. Shpilevskii, Yu. E. Andiya-Pravdivyi, S. G. Alekseeva, V. B. Grigoŕev, and V. I. Shvets, Bioorg. Khim., 24 (10), 787 (1998)
A. N. Kislitsyn, Khim. Drev., 3, 3 (1994).
V. Zuco, R. Supino, S. C. Righetti, L. Cleris, E. Marchesi, C. Gambacorti-Passerini, and F. Formelli, Cancer Lett., 175 (1), 17 (2002).
J. M. Pezzuto, T. K. DasGupta, M. L. Schmidt, K. M. Kuzmanoff, L. Ling-Indeck, and D. S. H. L. Kim, US Pat. No. 5,962,527, Oct. 5, 1999; Appl., Mar. 21, 1995.
A. P. Kaplun, Ju. E. Andija-Pravdivyi, S. V. Bureeva, L. V. Kozlov, and V. I. Shvets, RU Pat. No. 2,243,233, Dec. 27, 2004.
V. A. Levdanskii, A. V. Levdanskii, and B. N. Kuznetsov, Khim. Rastit. Syŕya, No. 1, 107 (2013).
V. I. Grishkovets, Chem. Nat. Compd., 35, 73 (1999).
E. E. Gilbert, Sulfonation and Related Reactions, Interscience Publishers, New York, 1965, 530 pp.
V. A. Levdansky, A. S. Kondracenko, A. V. Levdansky, B. N. Kuznetsov, L. Djakovitch, and C. Pinel, J. SFU Chem., 7, No. 2, 162 (2014).
V. A. Levdanskii, A. V. Levdanskii, and B. N. Kuznetsov, Chem. Nat. Compd., 50, 1029 (2014).
J. Kitajima, M. Shindo, and Y. Tanaka, Chem. Pharm. Bull., 38 (3), 714 (1990).
D. S. H. L. Kim, Z. Chen, V. T. Nguyen, J. M. Pezzuto, S. Qiu, and Z.-Z. Lu, Synth. Commun., 27 (9), 1607 (1997).
B. N. Kuznetsov, V. A. Levdanskii, S. A. Kuznetsova, and T. I. Kogai, Zh. SFU Khim., 4 (4), 408 (2011).
A. J. Gordon and R. A. Ford, The Chemisťs Companion, Wiley-Interscience, New York, 1972, 537 pp.
V. A. Levdanskii, A. V. Levdanskii, and B. N. Kuznetsov, Khim. Rastit. Syŕya, No. 4, 79 (2012).
Acknowledgment
The work was sponsored by the Russian Ministry of Education (Project RFMEF160714X0031). Instruments of the Krasnoyarsk Regional Center for Collective Use, SB, RAS, were used in the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2015, pp. 767–769.
Rights and permissions
About this article
Cite this article
Levdanskii, V.A., Levdanskii, A.V. & Kuznetsov, B.N. Sulfonation of Betulinic Acid by Sulfamic Acid. Chem Nat Compd 51, 894–896 (2015). https://doi.org/10.1007/s10600-015-1442-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-015-1442-1