A new lignan, named ginkgool (1), was isolated from the roots of Ginkgo biloba together with four known lignans, isolariciresinol (2), cycloolivil (3), pinoresinol (4), and sesamin (5). The new lignan was characterized as 4β-(5-hydroxy-3-methoxybenzyl)-2α-(4-hydroxy-3-methoxyphenyl)-3β-(hydroxymethyl)-tetrahydrofuran-4α-ol (1). The structures of the five compounds were determined on the basis of physicochemical and spectrometric data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ginkgo biloba L., known as “living fossil,” has been used as a traditional Chinese medicines for a long time [1]. Lots of studies have been carried out on the leaves of G. biloba, and many chemical constituents with significant bioactivities, such as terpene lactones and flavonoids, have been isolated. In the process of our study, a new lignan from the roots of G. biloba and four known compounds were isolated, whose structures were established primarily on the basis of spectroscopic techniques, including 1D and 2D NMR methods.
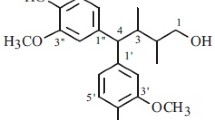
Compound 1 was obtained as white needle crystals. (–)-HR-ESI-MS confirmed the molecular formula to be C20H24O7 based on its ion peak at 375.1447 [M – H]– (calcd 375.1444). which indicated nine degrees of unsaturation. In its 1H NMR spectrum (Table 1), two typical peaks δ 3.43 (1H, d, J = 8.8 Hz) and 3.61 (1H, d, J = 8.9 Hz) and the typical signal at 4.59 (1H, d, J = 9.7 Hz) indicated that 1 was a tetrahydrofuran lignan. Signals at δ 7.03 (1H, d, J = 1.8 Hz), 6.68 (1H, d, J = 8.1 Hz), and 6.79 (1H, dd, J = 8.1, 1.8 Hz) indicated an AMX benzene system, and signals at δ 6.85 (1H, br.s), 6.65 (1H, s), and 6.65 (1H, s) suggested the presence of a meta-substituted phenyl group. Two phenolic hydroxyl signals at δ 8.73 (1H, s) and 8.59 (1H, s) and two methoxy groups at δ 3.74 and 3.73 (each 3H, s) were on each of the two aromatic rings. The 13C NMR spectrum of compound 1 (Table 1) showed 20 carbon signals, including two aromatic rings (δ 111.0–147.2), one oxymethine (δ 83.2), one oxygenated quaternary carbon (δ 80.5), two oxygenated methylenes (δ 76.1, 60.5), one methane carbon (δ 58.9),one methylene carbon (δ 39.1), and two methoxy groups (δ 55.5). This further suggested that compound 1 is a 7-O-9-type tetrahydrofuran lignan. The 1H NMR and 13C NMR data of compound 1 were completely assigned with the HMBC data.
In the HMBC spectra, the correlations (Fig. 1) of H-2 (δ 4.59) with C-3 (δ 58.9) and C-3a (δ 60.5) led to the connection of C-3a and C-3 of compound 1. The correlations of 4-OH (δ 4.57) with C-4 (δ 80.5) and C-5 (δ 76.1) indicated that the hydroxy was placed at C-4. The correlation of H-6′′ (δ 6.79) and H-2′′ (δ 7.03) with C-2 (δ 83.2) confirmed that C-2 was attached to the 1,3,4-substituted benzene ring. The HMBC correlations of the proton signals at H-2′ (δ 6.85) and H-4′ (δ 6.65) with C-4a (δ 39.1) showed that C-4a was attached to the 1,3,5-substituted benzene ring. The correlations of 3′′ -OCH3/C-3′′ and 3′-OCH3/C-3′ and all data mentioned above established the planar structure of compound 1.
The relative configuration was established by the ROESY correlations. The correlations (Fig. 1) of H-2 with H-3a and 4-OH with H-3 showed that H-3 and 4-OH were α-oriented and H2-3a, H2-4a, and H-2 were β-oriented (Fig. 1). To support this, we found that H-3 showed a NOE with H-2′′, proving the α-orientation of 4-OH and H-3. Thus, the structure of compound 1 was established as 4β-(5-hydroxy-3-methoxybenzyl)-2α-(4-hydroxy-3-methoxyphenyl)-3β-(hydroxymethyl)-tetrahydrofuran-4α-ol, named ginkgool, which was similar to (2R,3S,4S)-4-(4-hydroxy-3-methoxybenzyl)-2-(5-hydroxy-3-methoxyphenyl)-3-(hydroxymethyl)-tetrahydrofuran-3-ol from Saussurea cordifolia [2].
Compounds 2–5 were known ligans. Their structures were identified as isolariciresinol (2) [3], cycloolivil (3) [4], pinoresinol (4) [5], and sesamin (5) [6] by comparison of their spectroscopic data with those in the related literature.
Experimental
General. NMR spectra were measured on a Bruker AV 500M NMR spectrometer. ESI-MS and HR-ESI-MS were measured on an Agilent 6224 TOF LC/MS spectrometer. Silica gel (100–200 mesh, 200–300 mesh, Qingdao Haiyang Chemical Co., Ltd.) was used. All solvents were of analytical grade (Tianjin BOYOU Chemical Plant).
Plant Material. The roots of Ginkgo biloba were collected in 2007 from Jiangsu Province, China and identified by Prof. Xue-hua Song (China Pharmaceutical University). A voucher specimen was deposited at the Department of Natural Medicinal Chemistry, China Pharmaceutical University, Nanjing 210009, China.
Extraction and Isolation. The roots of Ginkgo biloba (5 kg) were extracted three times by 95% ethanol. The extracted solvent was concentrated by a rotary vacuum evaporator, then extracted with ethyl acetate after suspension in water. The ethyl acetate extract (109 g) was separated on a silica gel column eluted with a gradient of petroleum ether–acetone (1:0–0:1, v/v) to afford four fractions (A–D). Fractions B (60 g) was separated on ODS using 30, 50, 70, and 90% MeOH–water and MeOH in sequence to afford five fractions (B1–B5). Fraction B3 (6 g) was separated on a Sephadex LH-20 column eluted with MeOH to afford 1 (100.6 mg). Fraction B2 was further chromatographed on a silica gel column eluted with a gradient solvent system of CH2Cl2–MeOH to afford several subfractions. After repeated silica gel column chromatography (CH2Cl2–MeOH, petroleum ether–EtOAc, petroleum ether–acetone), Sephadex LH-20 gel column chromatography (MeOH), and ODS reverse-phase column chromatography (MeOH–H2O), we obtain the four known lignans.
Ginkgool (1). White needle crystals. For 1H and 13C NMR, see Table 1. HR-ESI-MS (negative) m/z [M – H]–375.1447 (calcd for C20H24O7, 375.1444).
Isolariciresinol (2). White granular crystals. 1H NMR (500 MHz, DMSO-d6,δ, ppm, J/Hz): 8.67 (1H, s, OH), 8.36 (1H, s, OH), 6.68 (1H, d, J = 8.0, H-6), 6.63 (1H, s, H-2), 6.60 (1H, s, H-2′), 6.49 (1H, d, J = 8.1, H-5), 6.10 (1H, s, H-5′), 3.74 (1H, d, J = 10.2, H-7), 3.70 (3H, s, OCH3), 3.69 (3H, s, OCH3), 3.56 (1H, m, H-9′), 3.45 (1H, m, H-9), 3.43 (1H, m, H-9′), 3.17 (1H, m, H-9), 2.65 (2H, m, H-7′), 1.84 (1H, m, H-8′), 1.62 (1H, m, H-8). 13C NMR (125 MHz, DMSO-d6, δ, ppm): 147.3 (C-3), 145.5 (C-3′), 144.6 (C-4), 144.1 (C-4′), 137.1 (C-1), 132.7 (C-6′), 127.1 (C-1′), 121.5 (C-6), 116.2 (C-5′), 115.2 (C-5), 113.3 (C-2), 111.8 (C-2′), 63.6 (C-9′), 59.8 (C-9), 55.6 (OCH3), 55.5 (OCH3), 45.9 (C-7, 8), 38.1 (C-8′), 32.2 (C-7′). ESI-MS m/z 378 [M + NH4]+.
Cycloolivil (3). White needle crystals. 1H NMR (500 MHz, DMSO-d6, δ, ppm, J/Hz): 8.69 (1H, s, OH), 8.32 (1H, s, OH), 6.70 (1H, d, J = 8.0, H-5), 6.65 (1H, d, J = 1.8, H-2), 6.55 (1H, s, H-2′), 6.53 (1H, dd, J = 8.0, 1.8, H-6), 6.05 (1H, s, H-5′), 4.88 (1H, t, J = 4.3, OH), 4.65 (1H, t, J = 5.8, OH), 4.28 (1H, s, OH), 3.85 (1H, d, J = 11.7, H-7), 3.70 (3H, s, OCH3), 3.69 (3H, s, OCH3), 3.61 (2H, m, H-9′), 3.35 (2H, m, H-9), 3.09 (1H, d, J = 16.6, H-7′), 2.44 (1H, d, J = 16.6, H-7′), 1.88 (1H, d, J = 11.7, H-8). 13C NMR (125 MHz, DMSO-d6, δ, ppm): 147.3 (C-3), 145.7 (C-3′), 144.7 (C-4), 143.9 (C-4′), 136.9 (C-1), 132.1 (C-1′), 125.1 (C-6′), 121.8 (C-6), 116.1 (C-5′), 115.3 (C-5), 113.4 (C-2), 112.3 (C-2′), 72.9 (C-8′), 68.0 (C-9′), 59.0 (C-9), 55.7 (OCH3), 55.5 (OCH3), 45.6 (C-8), 43.0 (C-7), 40.0 (C-7′). ESI-MS m/z 399 [M + Na].
Pinoresinol (4). Yellow gum. 1H NMR (500 MHz, CDCl3, δ, ppm): 6.88 (4H, d, J = 8.0, H-2, 5, 2′, 5′), 6.82 (2H, dd, J = 1.3, 8.0, H-6, 6′), 4.74 (2H, d, J = 4.5, H-7, 7′), 4.24 (2H, dd, J = 9.3, 6.9, H-9, 9′), 3.89 (6H, s, OCH3), 3.10 (2H, m, H-8, 8′). 13C NMR (75 Hz, CDCl3, δ, ppm): 146.7 (C-3, 3′), 145.2 (C-4, 4′), 132.9 (C-1, 1′), 118.9 (C-5, 5′), 114.3 (C-6, 6′), 108.6 (C-2, 2′), 85.7 (C-7, 7′), 71.6 (C-6, 6′), 55.9 (C-9, 9′) , 54.3 (C-8, 8′). ESI-MS m/z 357 [M – H]–.
Sesamin (5). White granular crystals. 1H NMR (300 MHz, CDCl3, δ, ppm, J/Hz): 6.86 (2H, d, J = 10.4, H-5, 5′), 6.84 (2H, s, H-2, 2′), 6.76 (2H, d, J = 8.2, H-6, 6′), 5.90 (4H, s, 2 × OCH2), 4.71 (2H, d, J = 4.1, H-7, 7′), 4.22 (2H, dd, J = 6.8, 9.0, H-9b, 9b′), 3.85 (2H, dd, J = 3.4, 9.2, H-9a, 9a′), 3.04 (2H, m, H-8, 8′). 13C NMR (75 MHz, CDCl3, δ, ppm): 148.0 (C-4, 4′), 147.1 (C-3, 3′), 135.1 (C-1, 1′), 119.3 (C-6, 6′), 108.2 (C-5, 5′), 106.5 (C-2, 2′), 101.0 (2×OCH2), 85.8 (C-7, 7′), 54.3 (C-8, 8′).
References
P. Yu and J. Liang, Chin. Chem. Lett., 23, 1165 (2012).
X. W. Li, Z. T. Guo, Y. Zhao, Z. Zhao, and J. F. Hu, Phytochemistry, 71, 682 (2010).
Y. Liu, S. J. Song, S. X. Xu, and X. H. Fu, J. Shenyang Pharm. Univ., 02, 101 (2003).
X. H. Zheng and L. Y. Kong, Chin. Trad. Herb. Drugs, 11, 964 (2002).
D. M. Zhang, L. H. Hu, W. C. Ye, and S. X. Zhao, Chin. J. Nat. Med., 2, 80 (2003).
Seon-Mi Seo, Hak-Ju Lee, Oh-Kyu Lee, Hyun-Jin Jo, Ha-Young Kang, Don-Ha Choi, Ki-Hyon Paik, and M. Khan, Chem. Nat. Compd., 44, 419 (2008).
Acknowledgment
This work was supported by the Natural Science Foundation of Jiangsu Province (No. BK2011628) in China and the College Graduate Research and Innovation Programs Funded Project of Jiangsu Province (CXLX11_0809).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2015, pp. 704–706.
Rights and permissions
About this article
Cite this article
Wei, Xl., Chen, Y., Chen, Xy. et al. A New Lignan from the Roots of Ginkgo biloba . Chem Nat Compd 51, 819–821 (2015). https://doi.org/10.1007/s10600-015-1424-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-015-1424-3