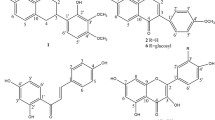
In the present study, phenolic constituents from the rhizomes of D. bonii were isolated and their antibacterial and antioxidant activities were determined. A new compound and eight known phenolic compounds were isolated from the ethanol extract of D. bonii. The structure of the new compound was characterized by spectroscopic analysis as 3-(5-hydroxymethyl)furan-2-yl)-2-phenylacrylaldehyde (9), along with chrysophanol (1), nobiletin (2), protocatechuic acid (3), protocatechualdehyde (4), isoliquiritigenin (5), rutin (6), nicotiflorin (7), and linocaffein (8). These compounds were isolated from this plant for the first time. Most of the compounds demonstrated antibacterial and antioxidant activities, with compound 4 demonstrating especially potent antioxidant effects (SC50 4.87 μg/mL) in the DPPH test.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The genus Drynaria is comprised of 19 species [1], of which seven are found in Vietnam [2]. Drynaria bonii Christ, a Vietnamese medicinal plant, has been traditionally used to heal bone fractures, stimulate the growth of hair, and treat tinnitus or deafness [2, 3]. Until now, there has been no literature on the chemical constituents isolated from this plant. In our continuing studies on the Drynaria species in Vietnam [4–6], this paper describes the isolation and structural determination of nine secondary metabolites from the ethanol fraction of the rhizomes of Drynaria bonii Christ. Bioassays showed that most of the metabolites had antibacterial activity using the disc diffusion method and antioxidant activity using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging test.
After a primary separation by column chromatography with silica gel (normal and reversed phase), the ethanol extract of D. bonii was partitioned successively with petroleum ether, chloroform, ethyl acetate, and methanol. After purifying by Sephadex LH-20 and ODS column chromatography, the petroleum ether, chloroform, ethyl acetate, and methanol fractions of D. bonii yielded nine compounds (1–9), one of which was a new compound (9).
Compound 9 was obtained as a dark brown powder. It reacted positively with FeCl3, proving that 9 was a phenolic compound. The molecular formula was established as C14H12O3 using HR-ESI-MS (the mass spectrum showed a pseudomolecular ion peak [M + Na]+ at m/z 251.0679; calcd for C14H12O3Na, 251.0684). The PMR spectrum of 9 displayed two doublet olefinic signals at δ 6.12 (1H, d, J = 3.5 Hz, H-3) and 6.35 (1H, d, J = 3.5 Hz, H-4), suggesting the presence of a 2,5-disubstituted furan ring, six aromatic protons at δ [7.16 (2H, m, H-2′, 6′), 7.45 (2H, m, H-3′, 5′), 7.44 (1H, m, H-4′), and 7.50 (1H, s, H-6)]. The singlet signal at δ 9.66 (1H, s, H-′) indicated the existence of an aldehyde in its structure. The 13C NMR spectrum showed 14 carbon signals, of which there was a carbonyl at δ 192.6 (C-β), two oxyaryl carbons at δ 149.2 (C-2) and 159.3 (C-5), eight methine aromatic carbons, and two quaternary aromatic carbons.
In the HMBC spectrum, two protons of a 2,5-disubstituted furan ring resonating at δ 6.12 (1H, d, J = 3.5 Hz) and 6.35 (1H, d, J = 3.5 Hz) were observed to be correlated with two oxygenated olefinic carbons at δ 149.2 and 159.3, indicating that these carbon signals were assigned to C-2 and C-5, respectively (Fig. 1). The correlation between hydroxymethyl protons (–CH2OH) at δ 4.33 (2H, s) with an oxygenated olefinic carbon at δ 159.3 (C-5) and a sp2 methine carbon at δ 110.0 in HMBC spectrum supported the assignment of the hydroxymethyl moiety at C-5 and the sp2 methine carbon at C-4. The correlation of a proton at δ 6.35 ppm (1H, d, J = 3.5 Hz) to C-4 (δ 110.0) in the HSQC spectrum confirmed that the proton was H-4. The singlet signal at δ 7.50 (1H, s), attributed to the methine carbon (δ 136.2) in the HSQC spectrum, gave HMBC correlations with C-3 (δ 117.5) and C-2 (δ 149.2), a quaternary sp2 carbon (δ 137.6), and a carbonyl carbon (δ 192.6), suggesting that the methine carbon was located at the C-2 position of the furan skeleton, and the singlet proton was assigned to H-6. Based on careful analysis of the 13C NMR and HMBC spectrum of 9, the six sp2 carbons, including five methine and one quaternary carbons, belong to a monosubstituted benzene ring [δH 7.44 (1H, m, H-4′), 7.16 and 7.45 (each 2H, m, H-2′, 6′ and H-3′, 5′, respectively); δC 128.0 (C-4′), 128.9 (C-2′, 6′), 128.3 (C-3′, 5′), and 133.5 (C-1′)]. Furthermore, the aldehyde proton at δ 9.66 (1H, s) correlated with two quaternary sp2 carbons at δ 137.6 and C-1′, proving that the aldehyde group was located at C-α (δ 137.6) and that C-α was attached to the benzene ring at C-1′. The cross-peak between a proton H-6 and aldehyde proton in the ROESY spectrum proved that the configuration of the isolated double bond was E. Therefore, compound 9 was identified as 3-(5-hydroxymethyl)furan-2-yl)-2-phenylacrylaldehyde (drynaran).
The known compounds 1–8 were identified as chrysophanol (1), nobiletin (2), protocatechuic acid (3), protocatechualdehyde (4), isoliquiritigenin (5), rutin (6), nicotiflorin (7), and linocaffein (8) by comparison of their NMR and MS data with those reported in the literature [7–14]. All compounds were isolated for the first time from the Drynaria bonii Christ plant. The isolates were assayed for their DPPH radical scavenging activity and antibacterial activity. Compounds 1–9 were not active against P. aeruginosa, B. subtilis, S. aureus, F. oxysporum, S. cerevisiae, and C. albicans. Compounds 1, 2, 4–6, and 9 had weak antibacterial activity (MIC 50 μg/mL). The MIC of compounds 1, 5, and 6 for E. coli and compounds 2, 4, and 9 for A. niger was 50 μg/mL. Compounds 3, 4, and 6 were strong scavengers of DPPH radicals (Table 1), with SC50 values of 13.12, 4.87, and 25.00 μg/mL, respectively.
Antioxidants have been reported to play a major role in ameliorating peroxidative damage induced by free radicals in membranes and tissues [15]. Therefore, they could be used as preventive and/or therapeutic agents against carcinogenesis, aging, and cardiovascular diseases [16].
Experimental
NMR spectra were recorded on a Bruker Avance-500 MHz using TMS as internal standard, and ESI-MS and HR-MS data were recorded on an Agilent 1100 LC-MSD Trap and a HR-FT-ICR MS Varian 920, respectively, at the Institute of Chemistry (Vietnam Academy of Science and Technology). TLC were performed on silica gel 60 F254 (Merck 1.05715), RP18 F254s (Merck). The zones were detected using UV at 254 or 365 nm or a solution of FeCl3–EtOH or H2SO4–EtOH. Column chromatography was performed on silica gel (240–430 mesh) or ODS or Sephadex LH-20.
Biological evaluations were carried out at the Experimental Biology Laboratory, Institute of Natural Products Chemistry, Vietnam Academy of Science and Technology.
Plant Material. The rhizomes of Drynaria bonii were collected in Vietnam in February 2013 and authenticated by the Centre of Medicinal Plants and Ginseng in Ho Chi Minh City (National Institute of Medicinal Material, Vietnam).
Extraction and Isolation. Dried rhizomes of D. bonii (2.0 kg) were extracted with 96% EtOH. The extract was evaporated to dryness (205.0 g) under reduced pressure. The ethanol extract was eluted on a silica gel column sequentially with petroleum ether, chloroform, ethyl acetate, and methanol.
The petroleum ether fraction (47.0 g) was chromatographed on a silica gel column and eluted with a gradient of petroleum ether–ethyl acetate (200:1 to 10:1) to yield five fractions. Fraction 3 was further subjected to silica gel column chromatography, followed by Sephadex LH-20 with MeOH–CHCl3 (50:50) as eluent to give 1 (5.0 mg). The chloroform fraction (18.7 g) was subjected to chromatography on silica gel with a petroleum ether–ethyl acetate gradient (100:1 to 50:1) and ethyl acetate–methanol (1:20) as eluent to yield six fractions. Fraction 3 was eluted on a silica gel column with petroleum ether–ethyl acetate (50:1) followed by Sephadex LH-20 to yield 9 (9.0 mg). The fourth fraction (6.0 g) was further passed over an ODS column with MeOH–H2O (3:1) as eluent, then purified on a Sephadex LH-20 column and eluted with (CHCl3–MeOH 50:50) to give 2 (5 mg). The ethyl acetate fraction (10.2 g) was subjected to column chromatography on silica gel as a stationary phase and eluted with petroleum ether–ethyl acetate–methanol mixtures of increasing polarity to yield five fractions. Fraction 3 (2.6 g) was rechromatographed on a silica gel column with chloroform–methanol–H2O (8:2:0.2) as eluent, then further chromatographed on Sephadex LH-20 to give 3 (32.0 mg), 4 (13.0 mg), and 5 (2.0 mg). The methanol fraction (110.0 g) was fractionated by column chromatography on silica gel using a mixture of petroleum ether–ethyl acetate–methanol with increasing polarity to yield six fractions. Fraction 3 was chromatographed over a silica gel column and eluted with CHCl3–MeOH–H2O (8:2:0.2 to 20:6:1) and ethyl acetate–methanol–water (20:1:1), then on a Sephadex LH-20 column using MeOH as eluent and on an ODS column with MeOH–H2O (50:50 to 20:80) to give 7 (12.0 mg) and 8 (10.0 mg). Fraction 5 was rechromatographed on silica gel with chloroform–methanol–water (20:6:1) and ethyl acetate–methanol–water (15:1:1), followed by Sephadex LH-20 with MeOH to afford 6 (15.0 mg).
Drynaran (9), dark yellow powder, mp 114–116 C (MeOH). HR-ESI-MS m/z 251.0679 [M + Na]+. 1H NMR (500 MHz, DMSO-d6, δ, ppm, J/Hz): 6.12 (1H, d, J = 3.5, H-3), 6.35 (1H, d, J = 3.5, H-4), 7.50 (1H, s, H-6), 4.33 (2H, s, H-7), 9.66 (1H, s, H-β), 7.16 (2H, m, H-2′, 6′), 7.45 (2H, m, H-3′, 5′), 7.44 (1H, m, H-4′). 13C NMR (125 MHz, DMSO-d6, δ, ppm): 149.2 (C-2), 117.5 (C-3), 110.0 (C-4), 159.3 (C-5), 136.2 (C-6), 55.7 (C-7), 137.6 (C-α), 192.6 (C-β), 133.5 (C-1′), 128.9 (C-2′, 6′), 128.3 (C-3′, 5′), 128.0 (C-4′).
References
T. Janssen and H. Schneider, Plant Syst. Evol., 252, 175 (2005).
D. T. Loi, Medicinal Plants and Remedy of Vietnam, Publisher of Medicine, Hanoi, 2004, p. 491.
P. H. Ho, Vietnamese Plants, 1, Publisher of Young, Hanoi, 2002, p. 83.
P. T. N. Trinh, N. C. Hao, and P. T. Thao, Vietnam J. Chem., 47 (4A), 463 (2009).
P. T. N. Trinh, N. C. Hao, and P. T. Thao, Vietnam J. Chem., 47 (4A), 468 (2009).
P. T. N. Trinh, L. T. Dung, N. C. Hao, and P. T. Thao, J. Med. Mat., 14 (6), 272 (2009).
C. Jie, Antonio M. Montanari, and Wilbur W. Widmer, J. Agric. Food Chem., 45, 364 (1997).
Cheng J. Ma, Gui S. Li, Da L. Zhang, Ke Liu, and Xiao Fan, J. Chromatogr. A, 1078, 188 (2005).
K. Kohei, N. Naonobu, and S. Masahiko, Phytochemistry, 62, 229 (2003).
Hyang B. Lee, K. Eun, Sang J. Park, Sang G. Bang, Taegilkim, and Dae W. Chung, J. Agric. Food Chem., 58, 4808 (2010).
Jing K. Tian, S. Feng, and Yi Y. Cheng, J. Asian Nat. Prod. Res., 8 (1–2), 35 (2006).
Michiko Tamano and Jugo Koketsu, Agric. Biol. Chem., 46 (7), 1913 (1982).
G. K. Jayaprakasha, Ohnishi-Kameyama Mayumi, Ono Hiroshi, Yoshida Mitsuru, and Rao L. Jaganmohan, J. Agric. Food Chem., 54, 1672 (2006).
De-wu Zhang, Sheng-jun Dai, Wan-hui Liu, and Gui-hai Li, Chin. J. Nat. Med., 8 (3), 196 (2010).
R. Carini, G. Poli, M. U. Diazini, S. P. Maddix, T. F. Slater, and K. H. Cheesman, Biochem. Pharmacol., 39, 1597 (1990).
K. F. Gey, Br. Med. Bull., 49, 679 (1993).
Acknowledgment
This research was funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 104.01-2012.39.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2015, pp. 414–416.
Rights and permissions
About this article
Cite this article
Trinh, P.T.N., Tri, M.D., An, N.H. et al. Phenolic Compounds from the Rhizomes of Drynaria bonii . Chem Nat Compd 51, 476–479 (2015). https://doi.org/10.1007/s10600-015-1318-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-015-1318-4