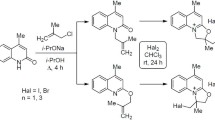
Mannich reaction of dihydroquercetin with a series of 1-aryl-6,7-dimethoxytetrahydroisoquinolines synthesized heterocyclic mono-and di-substituted conjugates of dihydroquercetin and N-hydroxymethyl isoquinoline derivatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Flavonoids belong to a large class of natural heterocyclic compounds, possess a broad spectrum of biological activity [1], and can be chemically modified to produce new pharmacologically active compounds. Dihydroquercetin (DHQ, 1) is currently one of the most common and available flavonoids [2, 3]. It is a unique natural free-radical acceptor and is recognized as a standard for very high antioxidant activity.
Mannich reactions with primary and secondary amines are known to produce high yields of DHQ aminomethyl derivatives [4–7]. It was shown that mono- and di-substituted products could be obtained depending on the reagent ratio and order of addition. It was established that the Mannich reaction could be carried out both with formation (isolation) of an intermediate complex of DHQ with secondary amines and in a three-component mixture [4, 5, 7]. Primarily DHQ 6-aminomethyl derivatives were obtained with an equimolar reagent ratio.
The goal of the present work was to synthesize mono-substituted DHQ derivatives. A series of 1-aryl-6,7-dimethoxytetrahydroisoquinolines (2a–h) that were prepared by us earlier were used as the amines [8, 9].
The reaction was carried out in i-PrOH at 20–25°C with DHQ–isoquinoline, 1:1 [4]. However, DHQ complexes with the isoquinolines (2a–h) were not observed. Addition of equimolar amounts of formaldehyde formed mono- and di-substituted DHQ products 3a–f and 5b and g, respectively. In addition, isoquinoline N-hydroxymethyl derivatives 4f–h appeared as side products.
The direction of the reaction (Scheme 1, A, B, or C) and product yield depended on the structure of the starting isoquinoline. Use of isoquinolines with electron-donating substituents (methoxy and methylenedioxy groups) gave derivatives 3a and c–f in good yields (pathway A) with DHQ reacting practically completely. The electron-accepting nitro group in isoquinoline 2b led to the formation of both mono- (3b) and di-substituted (5b) products (pathways A and C). Bromosubstituted isoquinoline 2g afforded only the di-substituted DHQ product 5g (pathway C).

Scheme 1
1-(2′ -Hydroxyphenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (2h) under the above conditions did not undergo a Mannich reaction. The only reaction product was hydroxymethyl derivative 4h. Starting DHQ was completely recovered. The hydroxymethyl derivatives were also obtained in small amounts (7–10%) for bromo-substituted isoquinolines 2f and 2g. Resonances of N–CH2–OH protons in PMR spectra of these compounds confirmed that 2-hydroxymethyl isoquinoline derivatives 4f–h had formed.
The synthesized compounds were characterized using IR and NMR spectroscopy. IR spectra of the conjugates showed strong absorption bands for hydroxyls (3435–3204 cm−1) and carbonyls (1639–1633 cm−1) of the DHQ fragment. PMR spectra exhibited resonances for H-3 at 4.35–4.50 ppm; doublets of doublets for H-2, 4.66–4.98; and singlets for H-8, 5.79–5.87. These were characteristic of the DHQ fragment. In addition, singlets for H-1 at 4.81–4.94 and aromatic H-5 at 6.76–6.87 and H-8 at 6.21–6.28 were characteristic of the isoquinoline part.
Resonances of H-3 and H-4 of the isoquinoline N–CH2–CH2 fragment in PMR spectra of 3a–f and 5b and g were assigned by analyzing COSY NMR spectra using 3d as an example. The 1H COSY spectrum of 3d contained a mutually coupled 4-spin system at 2.48–3.08 ppm that could only be methylene protons H-3 and H-4 in this structure. Three very weak cross-peaks at 2.5–3.1 ppm enabled the H-3 and H-4 resonances to be assigned. The two inner resonances of this group (δ 2.68 and 2.83 ppm) formed weak cross-peaks with the resonance for aromatic H-5 (6.46 ppm) and; therefore, were assigned to H-4. The resonance at 2.52 ppm showed a weak cross-peak at 3.57 ppm that was characteristic for one of the N–CH2–Ar methylene protons and; therefore, could be assigned to H-3.
Thus, amination of DHQ derivatives by 1-aryl-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolines produced mono- and di-substituted DHQ conjugates and hydroxymethyl derivatives of the starting isoquinolines.
EXPERIMENTAL
IR spectra were recorded from KBr pellets on a System 2000 FTIR instrument (PerkinElmer). PMR and 13C NMR spectra were recorded from DMSO-d6 solutions with HMDS internal standard on Unity-400 and Bruker AV-400 and DRX-500 spectrometers. TLC R f values were determined on LS 5/40 silica gel plates using CHCl3–MeOH (4:1, 1; 6:1, 2; 8:1, 3). Melting points of all synthesized compounds were determined on a Boetius apparatus. 1-Aryl-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolines (2a–h) were prepared by the literature method [8, 9].
Reaction of 1-Aryl-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolines (2a–h) with DHQ (1). General Method. A solution of 1-aryl-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (0.667 mmol) in i-PrOH (5 mL) was stirred, treated dropwise over 10 min with a solution of DHQ (0.2 g, 0.667 mmol) in i-PrOH (5 mL), held at 20–25°C for 0.5 h, and treated dropwise with formalin solution (30%, 0.06 mL, 0.667 mmol, d = 1.092). A precipitate began to form immediately. The mixture was left for 12 h. The course of the reaction was monitored by TLC. The precipitate was filtered off and rinsed with i-PrOH, hexane–Et2O (1:1), CHCl3–Et2O (1:1), and CHCl3. The precipitates polymerized in benzene and dioxane.
Equimolar amounts of DHQ (0.2 g, 0.667 mmol) and formalin solution (30%, 0.06 mL, 0.667 mmol) were used in the reactions described below.
2-(3,4-Dihydroxyphenyl)-6-{[6,7-dimethoxy-1-phenyl-3,4-dihydroisoquinolin-2(1 H )-yl]methyl}-3,5,7-trihydroxychroman-4-one (3a). 1-Phenyl-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (2a) produced 3a. Yield 0.32 g (81%), mp 162–166°C (i-PrOH), R f 0.59 (system 1). IR spectrum (KBr, νmax, cm−1): 3433 (OH), 1638 (C=O), 1516, 1449 (C=C), 1267 (C–O). 1H NMR spectrum (400 MHz, DMSO-d6, δ, ppm, J/Hz): a) DHQ fragment: 3.62 (1H, dd, J = 4.7, 14.2, N-CH2), 3.78 (1H, d, J = 14.2, NCH2), 4.45 (1H, dd, J = 5.6, 11.0, H-3), 4.92 (1H, t, J = 11.0, H-2), 5.67 (1H, br.s, 3-OH), 5.79 (1H, br.s, H-8), 6.70 (2H, s, H-5′,6′), 6.75 (1H, s, H-2′), 8.90, 8.94 (each 1H, br.s, 3′,4′-OH), 12.29 (1H, br.s, 5-OH), b) 1-phenyl-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline fragment: 2.64 (1H, m, Ha-3), 2.81 (2H, m, H-4), 2.96 (1H, m, He-3), 3.46 (3H, s, 7-OCH3), 3.71 (3H, s, 6-OCH3), 4.82 (1H, s, H-1), 6.21 (1H, br.s, H-8), 6.83 (1H, s, H-5), 7.15–7.38 (5H, m, Ar-H). 13C NMR spectrum (δ, ppm): a) DHQ fragment: 48.23 (CH2–N), 71.84 (C-3), 83.33 (C-2), 95.48 (C-8), 100.29 (C-6), 101.37 (C-4a), 115.42 (C-2′), 115.67 (C-5′), 119.72 (C-6′), 128.00 (C-1′), 145.25 (C-3′), 146.09 (C-4′), 161.01 (C-8a), 161.63 (C-5), 168.36 (C-7), 198.14 (C-4); b) 1-phenyl-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline fragment: 21.44 (C-4), 44.63 (C-3), 55.74 (6-OCH3), 55.84 (7-OCH3), 66.15 (C-1), 111.73 (C-8), 112.04 (C-5), 126.16 (C-8a), 127.83–128.90 (5C, Ph), 129.93 (C-4a), 147.44 (C-6), 148.06 (C-7).
Reaction of 1-(4′-Nitrophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline with DHQ. 1-(4′-Nitrophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (2b, 0.21 g, 0.667 mmol) produced a mixture of 3b and 5b, R f 0.63 and 0.57 (system 1). The yield of the mixture was 0.27 g (66%). Products 3b and 5b were purified by preparative chromatography over SiO2 using system 1.
2-(3,4-Dihydroxyphenyl)-6-{[6,7-dimethoxy-1-(4-nitrophenyl)-3,4-dihydroisoquinolin-2(1 H )-yl]methyl}-3,5,7-trihydroxychroman-4-one (3b), mp 178–182°C (i-PrOH), R f 0.63 (system 1), yield 36%. IR spectrum (KBr, νmax, cm−1): 3075 (OH), 2939 (CH2), 1639 (C=O), 1519 (NO2), 1463 (C=C), 1349 (C–NO2), 1265, 1122 (C–O). 1H NMR spectrum (500 MHz, DMSO-d6, δ, ppm, J/Hz): a) DHQ fragment: 3.68 (2H, resonance overlapped, N–CH2), 4.49 (1H, br.s, J = 11.0, H-3), 4.94 (1H, m, H-2), 5.78 (1H, br.s, 3-OH), 5.87 (1H, s, H-8), 6.74 (2H, s, H-5′, 6′), 6.78 (1H, s, H-2′), 9.00, 9.06 (each 1H, br.s, 3′, 4′-OH), 12.33 (1H, br.s, 5-OH); b) 1-(4′-nitrophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline fragment: 2.66 (1H, m, Ha-3), 2.83 (2H, m, H-4), 2.97 (1H, m, Ha-3), 3.51 and 3.74 (each 3H, s, 6, 7-OCH3), 4.94 (1H, s, H-1), 6.26 (1H, s, H-8), 6.86 (1H, s, H-5), 7.53 (2H, d, J = 8.5, H-2′, 6′), 8.20 (2H, d, J = 8.5, H-3′, 5′).
2-(3,4-Dihydroxyphenyl)-6,8- bis {[6,7-dimethoxy-1-(4-nitrophenyl)-3,4-dihydroisoquinolin-2(1 H )-yl]methyl}-3,5,7-trihydroxychroman-4-one (5b), mp 170–173°C (i-PrOH), R f 0.57 (system 1), yield 24%. IR spectrum (KBr, νmax, cm−1): 3435 (OH), 2924 (CH2), 1633 (C=O), 1519 (NO2), 1451 (C=C), 1348 (C–NO2), 1261, 1122 (C–O). 1H NMR spectrum (500 MHz, DMSO-d6, δ, ppm, J/Hz): a) DHQ fragment: 3.46 (4H, resonances overlapped, 2N–CH2), 4.46 (1H, d, J = 11.6, H-3), 4.81 (1H, d, J = 11.6, H-2), 5.75 (1H, br.s, 3-OH), 6.69 (3H, s, H-2′, 5′, 6′), 8.93, 9.06 (each 1H, br.s, 3′, 4′-OH), 12.37 (1H, br.s, 5-OH); b) two 1-(4′-nitrophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline fragments: 2.62 (2H, m, Ha-3, 3), 2.79 (4H, m, H-4, 4), 2.90 (2H, m, Ha-3, 3), 3.45, 3.47 (each 3H, s, 7, 7-OCH3), 3.69, 3.71 (each 3H, s, 6, 6-OCH3), 4.92, 4.93 (each 1H, br.s, H-1, 1), 6.18, 6.24 (each 1H, s, H-8, 8), 6.75, 6.83 (each 1H, s, H-5, 5), 7.29, 7.46 (each 2H, m, H-2′, 2′, 6′, 6′), 7.96 (4H, m, H-3′, 3′, 5′, 5′).
2-(3,4-Dihydroxyphenyl)-6-{[6,7-dimethoxy-1-(4-methoxyphenyl)-3,4-dihydroisoquinolin-2(1 H )-yl]methyl}-3,5,7-trihydroxychroman-4-one (3c). 1-(4′-Methoxyphenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (2c, 0.2 g, 0.667 mmol) afforded 3c. Yield 0.31 g (78%), mp 180–185°C (i-PrOH), R f 0.57 (system 2). IR spectrum (KBr, νmax, cm−1): 3423 (OH), 1642 (C=O), 1514, 1463 (C=C), 1255 (C–O). 1H NMR spectrum (500 MHz, DMSO-d6, δ, ppm, J/Hz): a) DHQ fragment: 3.67 (1H, dd, J = 4.8, 14.3, N–CH2), 3.82 (1H, d, J = 14.3, N–CH2), 4.48 (1H, dd, J = 5.5, 11.0, H-3), 4.96 (1H, t, J = 10.6, H-2), 5.81 (1H, s, H-8), 6.74 (2H, s, H-5′, 6′), 6.78 (1H, s, H-2′), 8.97 (2H, br.s, 3′, 4′-OH), 12.31 (1H, br.s, 5-OH); b) 1-(4′-methoxyphenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline fragment: 2.68 (1H, m, Ha-3), 2.85 (2H, m, H-4), 2.99 (1H, m, Ha-3), 3.51 (3H, s, 7-OCH3), 3.74 (6H, s, 6, 4′-OCH3), 4.82 (1H, s, H-1), 6.25 (1H, s, H-8), 6.87 (1H, s, H-5), 6.91 (2H, d, J = 8.5, H-3′, 5′), 7.10 (2H, d, J = 8.5, H-2′, 6′). 13C NMR spectrum (δ, ppm): a) DHQ fragment: 48.22 (CH2–N), 71.73 (C-3), 83.20 (C-2), 95.46 (C-8), 100.08, 101.00 (C-4a, 6), 115.32 (C-2′), 115.55 (C-5′), 119.55 (C-6′), 128.22 (C-1′), 145.14 (C-3′), 145.96 (C-4′), 160.79 (C-8a), 161.71 (C-5), 168.62 (C-7), 197.88 (C-4); b) 1-(4′-methoxyphenyl)-6,7- dimethoxy-1,2,3,4-tetrahydroisoquinoline fragment: 21.24 (C-4), 44.48 (C-3), 55.25 (6-OCH3), 55.66 (7-OCH3), 55.74 (4′-OCH3), 65.29 (C-1), 111.67 (C-8), 112.08 (C-5), 113.90 (C-2′), 113.90 (C-3′), 125.99 (C-1′), 128.05 (C-5′), 128.22 (C-6′), 130.97 (C-8a), 133.31 (C-4a), 147.40 (C-6), 148.01 (C-7), 158.87 (C-4′).
2-(3,4-Dihydroxyphenyl)-6-{[1-(3,4-methylenedioxyphenyl)-6,7-dimethoxy-3,4-dihydroisoquinolin-2(1 H )-yl]methyl}-3,5,7-trihydroxychroman-4-one (3d). 1-(3′,4′-Methylenedioxyphenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (2d, 0.21 g, 0.67 mmol) afforded 3d. Yield 0.34 g (83%), mp 176–180°C (i-PrOH), R f 0.34 (system 2). IR spectrum (KBr, δmax, cm−1): 3204 (OH), 1639 (C=O), 1516, 1488 (C=C), 1252 (C–O). 1H NMR spectrum (400 MHz, DMSO-d6, δ, ppm, J/Hz): a) DHQ fragment: 3.67 (1H, dd, J = 3.3, 14.3, N–CH2), 3.79 (1H, d, J = 14.3, N–CH2), 4.49 (1H, dd, J = 3.9, 11.1, H-3), 4.96 (1H, dd, J = 7.8, 11.1, H-2), 5.83 (1H, s, H-8), 6.74 (2H, s, H-5′, 6′), 6.87 (1H, s, H-2′), 9.00 (2H, br.s, 3′, 4′-OH), 12.33 (1H, br.s, 5-OH); b) 1-(3′4′-methylenedioxyphenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline fragment: 2.65 (1H, m, Ha-3), 2.82 (2H, m, H-4), 2.99 (1H, m, Ha-3), 3.52 (3H, s, 7-OCH3), 3.74 (3H, s, 6-OCH3), 4.77 (1H, s, H-1), 6.01 (2H, d, J = 3.4, 3′, 4′-OCH2O), 6.27 (1H, s, H-8), 6.68 (1H, s, H-2′), 6.69 (1H, d, J = 6.4, H-6′), 6.76 (1H, s, H-5), 6.88 (1H, d, J = 8.5, H-5′). 13C NMR spectrum (δ, ppm): a) DHQ fragment: 47.87 (CH2–N), 71.51 (C-3), 82.99 (C-2), 95.20 (C-8), 99.93, 101.09 (C-4a, 6), 115.09 (C-2′), 115.33 (C-5′), 119.37 (C-6′), 127.69 (C-1′), 144.92 (C-3′), 145.76 (C-4′), 160.67 (C-8a), 161.48 (C-5), 168.08 (C-7), 197.83 (C-4); b) 1-(3′4′-methylenedioxyphenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline fragment: 25.49 (C-4), 44.53 (C-3), 55.40 (6-OCH3), 55.48 (7-OCH3), 65.60 (C-1), 101.09 (C-7′), 107.78 (C-5′), 109.36 (C-2′), 111.37 (C-8), 111.64 (C-5), 123.28 (C-6′), 125.80 (C-1′), 127.99 (C-8a), 135.32 (C-4a), 146.68 (C-6), 147.12 (C-7), 147.31 (C-3′), 147.74 (C-4′).
2-(3,4-Dihydroxyphenyl)-6-{[1-(3,4-dimethoxyphenyl)-6,7-dimethoxy-3,4-dihydroisoquinolin-2(1 H )-yl]methyl}-3,5,7-trihydroxychroman-4-one (3e). 1-(3′,4′-Dimethoxyphenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (2e, 0.22 g, 0.667 mmol) afforded 3e. Yield 0.35 g (84%), mp 174–178°C (i-PrOH), R f 0.71 (system 2). IR spectrum (KBr, νmax, cm−1): 3434 (OH), 1641 (C=O), 1516, 1462, 1449 (C=C), 1261 (C–O). 1H NMR spectrum (500 MHz, DMSO-d6, δ, ppm, J/Hz): a) DHQ fragment: 3.70 (2H, resonance overlapped, N–CH2), 4.48 (1H, dd, J = 3.6, 11.0, H-3), 4.95 (1H, t, J = 10.9, H-2), 5.82 (1H, s, H-8), 6.74 (2H, s, H-5′, 6′), 6.87 (1H, s, H-2′), 8.98 (2H, br.s, 3′, 4′-OH), 12.36 (1H, br.s, 5-OH); b) 1-(3′,4′-dimethoxyphenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline fragment: 2.68 (1H, m, Ha-3), 2.85 (2H, m, H-4), 3.03 (1H, m, Ha-3), 3.51 (3H, s, 7-OCH3), 3.69 (3H, s, 6-OCH3), 3.74 (3H, s, 3′-OCH3), 3.75 (1H, s, 4′-OCH3), 4.47 (1H, d, J = 1.9, H-1), 6.28 (1H, s, H-8), 6.67 (2H, dd, J = 1.8, 8.2, H-6′), 6.77 (1H, s, H-5), 6.80 (1H, d, J = 1.8, H-2′), 6.91 (1H, d, J = 8.2, H-5′). 13C NMR spectrum (δ, ppm): a) DHQ fragment: 48.36 (CH2–N), 71.78 (C-3), 83.24 (C-2), 95.44 (C-8), 100.11, 101.18 (C-4a, 6), 115.35 (C-2′), 115.58 (C-5′), 119.59 (C-6′), 128.24 (C-1′), 145.17 (C-3′), 146.00 (C-4′), 160.84 (C-8a), 161.72 (C-5), 168.60 (C-7), 197.95 (C-4); b) 1-(3′,4′-dimethoxyphenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline fragment: 21.44 (C-4), 45.07 (C-3), 55.65 (6-OCH3), 55.67 (7-OCH3), 55.67 (3′-OCH3), 55.79 (4′-OCH3), 65.96 (C-1), 111.56 (C-8), 111.67 (C-5), 112.12 (C-6′), 113.27 (C-5′), 122.26 (C-2′), 125.99 (C-1′), 127.97 (C-8a), 133.88 (C-4a), 147.37 (C-6), 148.02 (C-7), 148.54 (C-3′), 148.78 (C-4′).
Reaction of 1-(6′-Bromo-3′,4′-dimethoxyphenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline with DHQ. 1-(6′-Bromo-3′,4′-dimethoxyphenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (2f, 0.27 g, 0.667 mol), DHQ (1, 0.2 g, 0.667 mmol), and formalin solution (30%, 0.06 mL, 0.667 mmol) afforded a mixture of 3f and 4f. The yield of the mixture was 0.31 g (65%), R f 0.67 and 0.71 (system 2). The products were purified by preparative chromatography over silica gel (system 2).
2-(3,4-Dihydroxyphenyl)-6-{[1-(6′-bromo-3′,4′-dimethoxyphenyl)-6,7-dimethoxy-3,4-dihydroisoquinolin-2(1 H )-yl]methyl}-3,5,7-trihydroxychroman-4-one (3f). Yield 50%, mp 168–172°C (i-PrOH), R f 0.67 (system 2). 1H NMR spectrum (400 MHz, CDCl3, δ, ppm, J/Hz): a) DHQ fragment: 3.70 (2H, s, NCH2), 4.33 (1H, d, J = 11.4, H-3), 4.66 (1H, br. resonance, H-2), 5.81 (1H, s, H-8), 6.72, 6.78 (each 1H, d, J = 7.7, H-5′, 6′), 6.88 (1H, s, H-2′); b) 1-(6′-bromo-3′,4′-dimethoxyphenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline fragment: 2.70, 3.03, 3.21 (4′, all m, H-3, 4), 3.59 (3H, s, 7-OCH3), 3.64 (3H, s, 6-OCH3), 3.81 (6H, s, 3′, 4′-OCH3), 5.13 (1H, s, H-1), 6.10 (1H, s, H-8), 6.56 (1H, s, H-2′), 6.73 (1H, s, H-5), 6.99 (1H, s, H-5′).
1-(6′-(6′-Bromo-3′,4′-dimethoxyphenyl)-2-hydroxymethyl-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (4f). Yield~10%, R f 0.71 (system 2). 1H NMR spectrum (400 MHz, DMSO-d6, δ, ppm, J/Hz): 2.79 (2H, br.s, H-4), 2.89 (2H, br.s, H-3), 3.68 (6H, s, 6, 7-OCH3), 3.71 (6H, s, 3, 4′-OCH3), 4.49 (1H, d, J = 11.0, N–CH2), 4.96 (1H, d, J = 11.0, N–CH2), 5.79 (1H, s, H-1), 6.68 (1H, s, H-8), 6.70 (1H, s, H-5), 6.74 (1H, s, H-2′), 6.87 (1H, s, H-5′).
Reaction of 1-(3′-Bromophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline with DHQ. 1-(3′-Bromophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (2g, 0.23 g, 0.667 mmol) afforded a mixture of 4g and 5g. The overall yield of the mixture was 0.19 g (44%), R f 0.42 and 0.79 (system 3).
1-(3′-Bromophenyl)-2-hydroxymethyl-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (4g). Yield ~10%, mp 268–272°C (MeOH), R f 0.42 (system 3). 1H NMR spectrum (400 MHz, CDCl3, δ, ppm, J/Hz): 2.86, 2.99, 3.07, 3.17 (each 1H, m, H-3, 4), 3.58 (2H, s, N-CH2), 3.61 (3H, s, 7-OCH3), 3.82 (3H, s, 6-OCH3), 4.83 (1H, br.s, OH), 5.24 (1H, s, H-1), 6.16 (1H, s, H-8), 6.58 (1H, s, H-5), 7.16 (2H, m, H-5′, 6′), 7.36 (1H, s, H-2′), 7.40 (1H, d, J = 6.7, H-4′).
2-(3,4-Dihydroxyphenyl)-6,8- bis {[6,7-dimethoxy-1-(3′-bromophenyl)-3,4-dihydroisoquinolin-2(1 H )-yl]methyl}-3,5,7-trihydroxychroman-4-one (5g). Yield 24%, mp 166–170°C (i-PrOH), R f 0.79 (system 3). 1H NMR spectrum (500 MHz, DMSO-d6, δ, ppm, J/Hz): a) DHQ fragment: 3.62, 3.70 (each 2H, m, 2N–CH2), 4.50 (1H, d, J = 11.5, H-3), 4.96 (1H, dd, J = 4.4, 11.3, H-2), 5.76 (1H, br.s, 3-OH), 6.74 (2H, s, H-5′, 6′), 6.77 (1H, s, H-2′), 9.0 (2H, br.s, 3′, 4′-OH), 12.36 (1H, br.s, 5-OH); b) two 1-(3′-bromophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline fragments: 2.64 (2H, m, Ha-3, 3), 2.81 (4H, m, H-4, 4), 2.94 (2H, m, Ha-3, 3), 3.50, 3.52 (each 3H, s, 7, 7-OCH3), 3.72, 3.74 (each 3H, s, 6, 6-OCH3), 4.83 (1H, s, H-1), 5.87 (1H, s, H-1), 6.22 (1H, s, H-8), 6.29 (1H, s, H-8), 6.72 (1H, s, H-5), 6.87 (1H, s, H-5), 7.19–7.33(4H, m, Ar–H), 7.41–7.49 (4H, m, Ar–H).
1-(2′-Hydroxyphenyl)-2-hydroxymethyl-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (4h). 1-(2′-Hydroxyphenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (2h, 0.19 g, 0.667 mmol) afforded 4h. Yield 0.17 g (44%), mp 201–203°C (i-PrOH), R f 0.54 (system 3). 1H NMR spectrum (400 MHz, CDCl3, δ, ppm, J/Hz): 2.62 (1H, m, Ha-4), 3.00 (2H, m, H-3), 3.13 (1H, m, Ha-4), 3.80 (3H, s, 7-OCH3), 3.88 (3H, s, 6-OCH3), 4.89 (1H, d, J = 11.0, N–CH2), 5.26 (1H, d, J = 11.0, N–CH2), 5.27 (1H, s, H-1), 6.59 (1H, s, H-8), 6.64 (2H, m, H-3′, 6′), 6.74 (1H, s, H-5), 7.03 (2H, m, H-4′, 5′).
References
R. B. Keller (ed.), Flavonoids: Biosynthesis, Biological Effects and Dietary Sources, Nova Science Publishers Inc., 2009, 347 pp.
V. A. Babkin, N. N. Ostroukhova, and N. N. Trofimova, Larch Biomass: From Chemical Composition to Innovative Products [in Russian], Izd. Sib. Otd. Ross. Akad. Nauk, Novosibirsk, 2011, 236 pp.
A. E. Weidmann, Eur. J. Pharm., 684, 19 (2012).
E. E. Nifant´ev, S. E. Mosyurov, T. C. Kukhareva, and L. K. Vasyanina, Dokl. Ross. Akad. Nauk, 451 (4), 404 (2013).
N. V. Kosheleva, E. I. Chernyak, S. V. Morozov, V. I. Vinogradova, Sh. Sh. Sagdullaev, N. D. Abdullaev, and I. A. Grigor´ev, Chem. Nat. Compd., 50, 443 (2014).
T. S. Kukhareva, V. A. Krasnova, M. P. Koroteev, G. Z. Kaziev, L. N. Kuleshova, A. A. Korlyukov, M. Yu. Antinin, and E. E. Nifant´ev, Zh. Org. Khim., 40 (8), 1237 (2004).
L. Cher, T.-S. Hu, J. Zhu, H. Wu, and Z.-J. Yao, Synth. Lett., 8, 1225 (2006).
Sh. N. Zhurakulov, V. I. Vinogradova, and M. G. Levkovich, Chem. Nat. Compd., 49, 70 (2013).
Sh. N. Zhurakulov, V. I. Vinogradova, I. Z. Zhumaev, and P. B. Usmanov, Dokl. Akad. Nauk Resp. Uzb., No. 3, 51 (2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2015, pp. 51–55.
Rights and permissions
About this article
Cite this article
Zhurakulov, S.N., Babkin, V.A., Chernyak, E.I. et al. Aminomethylation of 1-Aryl-6,7-Dimethoxy-1,2,3,4-Tetrahydroisoquinolines by Dihydroquercetin. Chem Nat Compd 51, 57–61 (2015). https://doi.org/10.1007/s10600-015-1203-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-015-1203-1