Abstract
Antipsychotic medications carry an established lifetime risk of metabolic syndrome. This retrospective chart review evaluated feasibility of a metabolic monitoring clinical decision support tool (CDST) for weight, lipid, blood glucose, and blood pressure management of 163 clients in an early psychosis outpatient clinic over 2 years. Each parameter had at least 98 (60.1%) clients with a recorded value, the most being documented for weight with 112 (68.7%) clients. CDST adherence ranged from at least 54.3–100% for non-pharmacologic interventions (e.g. clinic counseling, referral to health program or primary care) and at least 33.3–100% for pharmacologic interventions (e.g. metformin). Though no baseline cardiometabolic abnormalities were identified, dyslipidemia and obesity were later found in 37 (22.7%) and 35 (21.5%) clients, respectively. Only 14 (8.6%) clients were prescribed medications for cardiometabolic abnormalities by psychiatrists in the clinic. Increasing focus on physical health is needed to better this population’s long-term prognosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For patients with schizophrenia spectrum disorders, antipsychotic medications are a cornerstone of medical care to reduce the positive symptoms of schizophrenia and to improve psychosocial functioning (Leucht et al. 2012). Unfortunately, second-generation antipsychotics (SGAs) have been shown to cause significant weight gain and metabolic abnormalities (Correll et al. 2009; De Hert et al. 2011; Gohlke et al. 2012). Individuals deemed at ultra-high risk for psychosis also have reduced rates of physical activity and increased rates of tobacco use compared to healthy controls, often resulting from social withdrawal and lack of resources (Carney et al. 2016; Lederman et al. 2017). These persistent effects increase the risk of metabolic syndrome, defined by the Adult Treatment Panel III (ATP-III) report as at least three of the following: waist circumference > 102 cm (> 88 cm for females); high-density lipoprotein cholesterol < 40 mg/dL (< 50 mg/dL for females); triglycerides ≥ 150 mg/dL; blood pressure ≥ 130/≥ 85 mm Hg; and fasting blood glucose ≥ 110 mg/dL (National Cholesterol Education Program 2002). Metabolic syndrome is a strong predictor of coronary heart disease and diabetes mellitus, disease states that are a leading cause of mortality in individuals with psychiatric disorders (Hennekens et al. 2005; Olfson et al. 2015).
While antipsychotic medications, diet, exercise, and social determinants of health (e.g. loss of employment and thereby a stable income source) primarily confer the risk of developing cardiometabolic issues, these medical problems may begin well before psychiatric treatment due to schizophrenia spectrum pathophysiology. Multiple studies have identified various metabolic abnormalities in patients with first-episode psychosis (FEP), with some finding pro-inflammatory marker levels in the peripheral blood of these patients at similar quantities to blood levels of patients with a chronic schizophrenia spectrum disorder in acute exacerbations (Balotsev et al. 2017; Garcia-Rizo et al. 2017; Miller et al. 2011; Misiak et al. 2016). FEP patients have also been shown to develop lipid and blood glucose irregularities before or in the first weeks of starting pharmacologic interventions, typically in conjunction with weight gain (Archie et al. 2015; Horsdal et al. 2017; Jensen et al. 2017; O’Donoghue et al. 2014; Perez-Iglesias et al. 2007, 2009; Petrikis et al. 2015; Pillinger et al. 2017; Saddichha et al. 2008). Though a causal relationship between psychiatric disorders and metabolic disease has not been definitively proven, a growing body of literature on this issue underscores the importance of early screening and interventions.
Existing gaps in mental health resources lead to challenges in addressing these cardiometabolic abnormalities early and regularly in FEP patient care. Medical literature suggests that a majority of psychiatrists are aware of these abnormalities but do not often perform necessary physical examinations and lab monitoring due to a desire to delegate to other healthcare professionals (e.g. pharmacists and nursing staff), time and equipment restraints, lack of confidence in managing physical health issues, or some combination of these factors (Cormac et al. 2004; Murray et al. 2015; Schneiderhan et al. 2009). While some guidelines provide recommendations for ongoing monitoring in patients with chronic schizophrenia spectrum disorders, there is no consensus between them on how to approach this monitoring in patients with FEP (American Diabetes Association 2004; Buchanan et al. 2010; Cooper et al. 2016; Gothefors et al. 2010; Lambert and Chapman 2004; Pringsheim et al. 2011; Woo et al. 2005).
The United States’ National Institute of Mental Health created the Recovery After an Initial Schizophrenia Episode Early Treatment Program (RAISE-ETP) research initiative in 2009 to improve FEP patients’ medical services and long-term prognosis (Correll et al. 2014). To approach these goals in a structured manner, researchers developed and implemented the NAVIGATE program in 34 clinic sites across 21 states (Mueser et al. 2015). NAVIGATE is a coordinated specialty care model focused on providing patients in the early stages of a schizophrenia spectrum disorder with individualized medication management, cognitive-behavioral therapy, family education, and employment and education support through a core group of team members. Physical health is maintained as a priority through a prescriber’s continuous metabolic monitoring and a clinician’s nutrition and exercise education modules. How these NAVIGATE interventions are carried out is determined by the enrolled clinic site. This study’s location is based upon NAVIGATE’s model, though it is not officially part of the NAVIGATE program.
The primary objective of this study was to evaluate the completion of such cardiometabolic interventions at a coordinated specialty care clinic organized similarly to the NAVIGATE model through a retrospective chart review of enrolled clients. The authors claim no known conflicts of interest with regards to this study.
Methods
Design
The study was conducted at the Eskenazi Health Midtown Community Mental Health Prevention and Recovery Center for Early Psychosis (PARC), a team-based community clinic in Indianapolis, Indiana, for individuals first diagnosed with a schizophrenia spectrum disorder. PARC is funded through a state mental health block grant by the federal Substance Abuse and Mental Health Services Administration (SAMHSA). While PARC closely follows the NAVIGATE model, it has notable differences in its structure: more defined roles for a clinical pharmacist, nurse, and case manager; more informal family involvement; and less explicitly focused on resilience with psychotherapy. The principal staff of PARC includes three attending psychiatrists, one psychiatric pharmacist, two registered nurses, one clinical psychologist, and five case managers/therapists. The study received ethical approval from the Indiana University Purdue University Indianapolis Institutional Review Board under an exempt review. The study enrolled subjects from a list of current and past clients engaged with PARC between the months of July 2013 and June 2015.
Subject data was collected from electronic medical record (EMR) systems on demographics; medications prescribed, specifically antipsychotics and those used to treat metabolic abnormalities; weight; blood pressure; hemoglobin A1c (HgbA1c), low-density-lipoprotein (LDL) direct or lipid profile; and referral status to a primary care provider or other healthcare services. Body mass index (BMI) was calculated manually when subjects had a documented height. Subjects were considered to have a cardiometabolic comorbidity if they met diagnostic criteria for obesity per BMI threshold of ≥ 30 kg/m2 (National Institutes of Health 1998); hypertension per systolic and diastolic blood pressure thresholds of ≥ 140 mm Hg and ≥ 90 mm Hg, respectively (James et al. 2014); dyslipidemia per cholesterol thresholds of LDL ≥ 100 mg/dL, total cholesterol ≥ 200 mg/dL, HDL < 40 mg/dL [< 50 mg/dL for females], and triglycerides ≥ 200 mg/dL (Jacobson et al. 2015); and diabetes per HgbA1c threshold of ≥ 6.5% (American Diabetes Association 2016). If subjects’ psychiatric diagnosis changed during the study period, the most frequently documented diagnosis during the study period was chosen for data collection.
Subjects
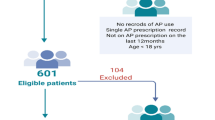
Clients were included in the study if they met all of the following inclusion criteria according to EMR information: (1) at least 18 years old; (2) seen for at least one visit with a PARC provider between July 1, 2013, and June 30, 2015; (3) diagnosis of schizophrenia, schizoaffective disorder, schizophreniform disorder, or psychosis not otherwise specified; (4) prescribed at least one antipsychotic medication; and (5) had lab results (i.e. weight, blood pressure, HgbA1c, LDL or lipid panel) provided by Eskenazi Health or from an outside health-system provider documented in the EMR. Clients prescribed a first-generation antipsychotic (FGA) were also included in the study due to potential cardiometabolic risks regardless of treatment. Clients visiting PARC during the study period were excluded if they were not prescribed an antipsychotic or did not fulfill the inclusion criteria related to age and diagnosis.
Clinical Decision Support Tool
To promote the holistic care of PARC clients, the clinic’s psychiatrists and psychiatric pharmacist created a clinical decision support tool (CDST) to aid clinicians in identifying and resolving medication side effects and cardiometabolic risk factors (Table 1). The CDST, known as “Fit Happens,” focuses on the parameters of weight, lipids, blood glucose, blood pressure, tobacco use, and sexual function. Under each of these parameters, clinicians can locate information on standard and intensive monitoring practices, non-pharmacologic interventions, and pharmacologic treatments according to vital sign and lab value abnormalities. The CDST was implemented clinic-wide through posted flyers and educational in-services, with an expectation that all providers would be 100% compliant with the recommended monitoring practices and interventions. This study evaluated those parameters targeting the metabolic health of PARC clients: weight, lipids, blood glucose, and blood pressure.
Statistical Analyses
Descriptive statistics were used for analysis, with medians and interquartile ranges reported to reflect the non-parametric nature of obtained data. Statistical analyses were performed using SPSS 24.0 (SPSS, Inc., Chicago, IL).
Results
Demographics
Of the 202 PARC clients on the initial census during the studied date range, 163 clients met the study’s inclusion criteria (Table 2). The study consisted of 90 subjects with schizophrenia (55.2%), 45 with psychosis not otherwise specified (27.6%), 19 with schizophreniform disorder (11.7%), and 9 with schizoaffective disorder (5.5%). Most subjects were male and of black ethnicity, with a median age of 22 (20–25) years. Approximately one-third of subjects reported tobacco use, and 47 subjects admitted to illicit drug use (primarily marijuana). Nearly one-fourth of subjects also met diagnostic criteria for dyslipidemia or obesity at some point in the study, with lesser degrees of hypertension and diabetes mellitus; no subjects met criteria for a cardiometabolic abnormality according to baseline data. Most subjects received SGAs that had both oral and long-acting injectable formulations: paliperidone (41.7%), risperidone (40.5%), olanzapine (33.8%), and aripiprazole (11%). Less than 7% of the study population used a FGA.
Because of smaller sample sizes in select genders, psychiatric diagnoses, and prescribed antipsychotic medications, further statistical analyses were performed on the whole study population rather than on subgroups.
CDST Feasibility
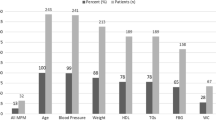
To better understand how metabolic monitoring and interventions were performed in the setting of an established CDST, vital sign and lab value completion was described as a percentage of 163 subjects (Table 3). Approximately two-thirds of subjects had at least one recorded weight, lipid, blood glucose, or blood pressure during the study period, with the most frequently documented parameter being weight (68.7%). No documentation of waist circumferences was found through chart review. Clinic counseling was completed with 36.8% of subjects at least once during the study, closely followed by primary care provider referrals (34.4%). PARC clinicians only made pharmacologic interventions in 9.2% of subjects, with all but two of these cases involving metformin. No statin medications were initiated as allowed by the CDST, nor were any antihypertensive medications.
Table 4 describes how often subjects with a vital sign or lab value meeting the “Trouble” or “Further Intervention” criteria were appropriately treated according to CDST recommendations; the smaller number of subjects in the “Further Intervention” category reflects the lesser severity of metabolic abnormalities seen in this FEP population over a 2-year period. Adherence was defined as “partially compliant” if at least one of the indicated interventions was performed, whereas “full compliance” indicated completion of all suggested interventions. In those subjects requiring initial interventions, nine of ten subjects were partially compliant with blood pressure management, though none were deemed fully compliant due to lack of hydrochlorothiazide use. All four subjects requiring initial interventions for lipids were at least partially compliant, and at least 50% of subjects with concerning weight (n = 71) and blood glucose (n = 35) values were partially or fully compliant as well. In subjects with severe abnormalities requiring further interventions, the highest likelihood of full compliance was observed for weight (n = 37) and blood glucose (n = 2) due to metformin use. Further interventions for lipids and blood pressure were limited by lack of indicated medication prescribing.
Discussion
This retrospective study considered cardiometabolic monitoring practices in an outpatient, community FEP clinic for 2 years under the guidance of a thorough CDST. None of the subjects upon entry to PARC met diagnostic criteria for a cardiometabolic disorder, though worsening trends were noted over time for weight and lipids in particular; this is comparable to baseline data from the Tolerability and Efficacy of Antipsychotics (TEA) trial, which included subjects aged 12–17 years with FEP (Jensen et al. 2017). A larger proportion of individual clinic visit documentation was comprised of weight and blood pressure (36.3–41%) than lipids and blood glucose (8.6–9.2%). The former range is bolstered by nursing staff documentation of weight and blood pressure values prior to each administration of a long-acting injectable antipsychotic, thereby exceeding CDST requirements. The latter range is hampered by a lack of point-of-care lab devices in PARC, meaning that clients must travel to a different area of the hospital for lipids and blood glucose tests to be drawn.
This study also assessed CDST adherence according to recommended interventions. Most subjects with later BMI values classified as “overweight” or “obese” were managed appropriately, paralleling the percentage of subjects that received clinic counseling (36.8%). Blood pressure, lipids, and blood glucose abnormality management were also fairly CDST-compliant, though few subjects were started or adjusted on a pharmacotherapy: 11 (6.7%) subjects with a medication for diabetes mellitus, 2 (1.2%) subjects with a medication for dyslipidemia, and no subjects with an antihypertensive. Such low medication prescribing, particularly of hydrochlorothiazide and pravastatin as the CDST allowed, is likely a reflection of PARC clinicians’ preferences for connecting their clients with a primary care provider instead (34.4%). While this study’s medication use data is comparable to RAISE-ETP treatment data, it still demonstrates an area for improvement in PARC’s cardiometabolic care (Correll et al. 2014).
Previous studies have also evaluated the frequency of cardiometabolic monitoring in FEP services. Crabb et al. (2009) carried out a retrospective case note audit of metabolic monitoring for 90 patients in an outpatient Scottish early psychosis intervention service; investigators found low initial screening rates for BMI (27%), fasting lipid profile (28%), fasting blood glucose (56%), and blood pressure (64%). Thompson et al. (2011) analyzed how providing local guidelines, educational seminars, paper monitoring sheets in patient files, and clinical assessment equipment would impact monitoring rates of 86 patients within an Australian youth mental health service spanning inpatient and outpatient settings. While baseline screening rates for weight, lipids, blood pressure, and blood glucose ranged from 74.4 to 84.9%, the 6-month post-intervention range decreased to 24.4% (blood glucose) to 41.6% (blood pressure). A study of United States community mental health centers also found most children on antipsychotics were still not receiving adequate cardiometabolic monitoring for lipids (31–33.1%) and blood pressure (49%) after a 3-year quality improvement program (Cotes et al. 2017). Finally, Carney et al. (2015) recently reiterated poor monitoring concerns in a retrospective review of 40 ultra-high risk patients seen by a United Kingdom early psychosis detection and intervention service, as no individual metabolic parameter was documented in more than 3 (7.5%) subjects. The percentage of subjects that received minimum screening and tracking of individual parameters of the “Fit Happens” CDST ranged from 60.1 to 68.7%, which is better than or comparable to rates observed in these prior studies.
Although this study adds insightful findings to the FEP literature, it is not without some limitations. The study was retrospective and without comparator groups, making it difficult to discern targets for correlation statistics. This study also occurred at one site, which decreases its external validity. Data collection was complicated by the health-system not having a single, unified EMR system, necessitating the synthesis of data from multiple sources. PARC’s primary EMR system did not have search functionality, making it difficult for investigators to discern CDST adherence according to recommended screening and tracking frequencies. The primary EMR system also did not have the capability to automate CDST interventions (i.e. alerting to perform physical assessments, ordering lab tests, prescribing medications for cardiometabolic abnormalities, initiating necessary referrals) due to software limitations. Having lab tests performed outside of the clinic added the possibility of client non-adherence despite a provider’s metabolic monitoring recommendations, a potential confounder that was unable to be accounted for in reported monitoring rates. Because the CDST had not been updated since its creation in 2009, it also may not have most accurately reflected prescriber intervention preferences (e.g. primary care provider referrals over direct pharmacotherapy initiations) and commonly performed clinic measurements (e.g. weight and BMI over waist circumference) during the study period.
It should be noted that this study only evaluated feasibility of an ongoing CDST, as data on monitoring practices at PARC prior to implementation of the “Fit Happens” CDST was not obtained. Limitations in data collection also prevent conclusions about the CDST’s effectiveness, with only baseline vital signs and lab values reported. Both are important areas for future research if practitioners are to better understand the clinical impact of such a tool on patients with FEP. Nonetheless, the minimum monitoring rates for this particular CDST are encouraging when compared to rates for similar FEP interventions, as well as to estimated rates up to 28.5% for lipids and 50% for blood glucose in pediatric Medicaid recipients using antipsychotics (Edelsohn et al. 2015; Morrato et al. 2010).
Conclusion
This retrospective study demonstrates that while a regimented CDST is feasible in a FEP clinic, additional quality improvement measures are needed for stronger provider adherence to evidence-based metabolic screening and intervention guidelines. Periodic reviews of such a CDST by all clinic staff can help ensure that included parameter guidelines remain relevant and appropriate, just as integrating primary care with mental health services can make completing parameter recommendations more realistic.
References
American Diabetes Association. (2016). Standards of medical care in diabetes—2016. Diabetes Care, 39(Suppl 1), S1–S112.
American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, & North American Association for the Study of Obesity. (2004). Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care, 272(2), 596–601.
Archie, S., Zangeneh-Kazemi, A., & Akhtar-Danesh, N. (2015). First-episode affective psychosis and lipid monitoring: Survival analysis of the first abnormal lipid test. Early Intervention in Psychiatry, 9(6), 507–511.
Balotsev, R., Haring, L., Koido, K., Leping, V., Kriisa, K., Zilmer, M., … Vasar, E. (2017). Antipsychotic treatment is associated with inflammatory and metabolic biomarkers alterations among first-episode psychosis patients: A 7-month follow-up study. Early Intervention in Psychiatry. https://doi.org/10.1111/eip.12457.
Buchanan, R. W., Kreyenbuhl, J., Kelly, D. L., Noel, J. M., Boggs, D. L., Fischer, B. A., … Schizophrenia Patient Outcomes Research Team (PORT). (2010). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophrenia Bulletin, 36(1), 71–93.
Carney, R., Bradshaw, T., & Yung, A. R. (2015). Monitoring of physical health in services of young people at ultra-high risk of psychosis. Early Intervention in Psychiatry. https://doi.org/10.1111/eip.12288.
Carney, R., Cotter, J., Bradshaw, T., Firth, J., & Yung, A. R. (2016). Cardiometabolic risk factors in young people at ultra-high risk for psychosis: A systematic review and meta-analysis. Schizophrenia Research, 170(2–3), 290–300.
Cooper, S. J., Reynolds, G. P., Barnes, T. R. E., England, E., Haddad, P. M., Heald, A., … Smith, J. (2016). BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. Journal of Psychopharmacology, 30(8), 717–748.
Cormac, I., Martin, D., & Ferriter, M. (2004). Improving the physical health of long-stay psychiatric in-patients. Advances in Psychiatric Treatment, 10(2), 107–115.
Correll, C. U., Manu, P., Olshanskiy, V., Napolitano, B., Kane, J. M., & Malhotra, A. K. (2009). Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA: The Journal of the American Medical Association, 302(16), 1765–1773.
Correll, C. U., Robinson, D. G., Schooler, N. R., Brunette, M. F., Mueser, K. T., Rosenheck, R. A., … Kane, J. M. (2014). Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: Baseline results from the RAISE-ETP study. JAMA Psychiatry, 71(12), 1350–1363.
Cotes, R. O., Fernandes, N. K., McLaren, J. L., McHugo, G. J., Bartels, S. J., & Brunette, M. F. (2017). Improving cardiometabolic outcomes of children on antipsychotics. Journal of Child and Adolescent Psychopharmacology. https://doi.org/10.1089/cap.2017.0034.
Crabb, J., McAllister, M., & Blair, A. (2009). Who should swing the stethoscope? An audit of baseline physical examination and blood monitoring on new patients accepted by an early intervention in psychosis team. Early Intervention in Psychiatry, 3(4), 312–316.
De Hert, M., Dobbelaere, M., Sheridan, E. M., Cohen, D., & Correll, C. U. (2011). Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: A systematic review of randomized, placebo controlled trials and guidelines for clinical practice. European Psychiatry, 26(3), 144–158.
Edelsohn, G. A., Parthasarathy, M., Terhorst, L., Karpov, I. O., & Schuster, J. (2015). Measurement of metabolic monitoring in youth and adult Medicaid recipients prescribed antipsychotics. Journal of Managed Care and Specialty Pharmacy, 21(9), 769–777.
Garcia-Rizo, C., Casanovas, M., Fernandez-Egea, E., Oliveira, C., Mesequer, A., Cabrera, B., … Bernardo, M. (2017). Blood cell count in antipsychotic-naive patients with non-affective psychosis. Early Intervention in Psychiatry. https://doi.org/10.1111/eip.12456.
Gohlke, J. M., Dhurandhar, E. J., Correll, C. U., Morrato, E. H., Newcomer, J. W., Remington, G., … Allison, D. B. (2012). Recent advances in understanding and mitigating adipogenic and metabolic effects of antipsychotic drugs. Frontiers in Psychiatry, 3, 62.
Gothefors, D., Adolfsson, R., Attvall, S., Erlinge, D., Jarbin, H., Lindstrom, K., … Swedish Psychiatric Association. (2010). Swedish clinical guidelines: Prevention and management of metabolic risk in patients with severe psychiatric disorders. Nordic Journal of Psychiatry, 64(5), 294–302.
Hennekens, C. H., Hennekens, A. R., Hollar, D., & Casey, D. E. (2005). Schizophrenia and increased risks of cardiovascular disease. American Heart Journal, 150(6), 1115–1121.
Horsdal, H. T., Benros, M. E., Kohler-Forsberg, O., Krogh, J., & Gasse, C. (2017). Metabolic profile at first-time schizophrenia diagnosis: A population-based cross-sectional study. Neuropsychiatric Disease and Treatment, 13, 621–630.
Jacobson, T. A., Ito, M. K., Maki, K. C., Orringer, C. E., Bays, H. E., Jones, P. H., … Brown, W. V. (2015). National Lipid Association recommendations for patient-centered management of dyslipidemia: Part 1 – full report. Journal of Clinical Lipidology, 9(2), 129–169.
James, P. A., Oparil, S., Carter, B. L., Cushman, W. C., Dennison-Himmelfarb, C., Handler, J., … Ortiz, E. (2014). 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA: The Journal of the American Medical Association, 311(5), 507–520.
Jensen, K. G., Correll, C. U., Ruda, D., Klauber, D. G., Stentebjerg-Olesen, M., Fagerlund, B., … Pagsberg, A. K. (2017). Pretreatment cardiometabolic status in youth with early-onset psychosis: Baseline results from the TEA trial. Journal of Clinical Psychiatry. https://doi.org/10.4088/JCP.15m10479.
Lambert, T. J., Chapman, L. H. & Consensus Working Group. (2004). Diabetes, psychotic disorders, and antipsychotic therapy: A consensus statement. Medical Journal of Australia, 181(10), 544–548.
Lederman, O., Rosenbaum, S., Maloney, C., Curtis, J., & Ward, P. B. (2017). Modifiable cardiometablic risk factors in youth with at-risk mental states: A cross-sectional pilot study. Psychiatry Research. https://doi.org/10.1016/j.psychres.2017.08.034.
Leucht, S., Tardy, M., Komossa, K., Heres, S., Kissling, W., Salanti, G., & Davis, J. M. (2012). Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: A systematic review and meta-analysis. The Lancet, 379(9831), 2067–2071.
Miller, B. J., Buckley, P., Seabolt, W., Mellor, A., & Kirkpatrick, B. (2011). Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biological Psychiatry, 70(7), 663–671.
Misiak, B., Wisniewski, J., Fleszar, M. G., & Frydecka, D. (2016). Alterations in L-arginine metabolism in first-episode schizophrenia patients: Further evidence for early metabolic dysregulation. Schizophrenia Research, 178(1–3), 56–57.
Morrato, E. H., Nicol, G. E., Maahs, D., Druss, B. G., Hartung, D. M., Valuck, R. J., … Newcomer, J. W. (2010). Metabolic screening in children receiving antipsychotic drug treatment. Archives of Pediatrics and Adolescent Medicine, 164(4), 344–351.
Mueser, K. T., Penn, D. L., Addington, J., Brunette, M. F., Gingerich, S., Glynn, S. M., … Kane, J. M. (2015). The NAVIGATE program for first-episode psychosis: Rationale, overview, and description of psychosocial components. Psychiatric Services, 66(7), 680–690.
Murray, J., Baillon, S., Bruce, J., & Velayudhan, L. (2015). A survey of psychiatrists’ attitudes toward the physical examination. Journal of Mental Health, 24(4), 249–254.
National Cholesterol Education Program. (2002). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III): Final report. Circulation, 106(25), 3143–3421.
National Institutes of Health, & National Heart, Lung, and Blood Institute. (1998). Clinical guidelines on the identification, evaluation and treatment of overweight and obesity in adults: The evidence report. Obesity Research, 6(Suppl 2), 51S–209S.
O’Donoghue, B., Schafer, M. R., Becker, J., Papageorgiou, K., & Amminger, G. P. (2014). Metabolic changes in first-episode early-onset schizophrenia with second-generation antipsychotics. Early Intervention in Psychiatry, 8(3), 276–280.
Olfson, M., Gerhard, T., Huang, C., Crystal, S., & Stroup, T. S. (2015). Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry, 72(12), 1172–1181.
Perez-Iglesias, R., Crespo-Facorro, B., Amado, J. A., Garcia-Unzueta, M. T., Ramirez-Bonilla, M. L., Gonzalez-Blanch, C., … Vazquez-Barquero, J. L. (2007). A 12-week randomized clinical trial to evaluate metabolic changes in drug-naïve, first-episode psychosis patients treated with haloperidol, olanzapine, or risperidone. Journal of Clinical Psychiatry, 68(11), 1733–1740.
Perez-Iglesias, R., Mata, I., Pelayo-Teran, J. M., Amado, J. A., Garcia-Unzueta, M. T., Berja, A., … Crespo-Facorro, B. (2009). Glucose and lipid disturbances after 1 year of antipsychotic treatment in a drug-naïve population. Schizophrenia Research, 107(2–3), 115–121.
Petrikis, P., Tigas, S., Tzallas, A. T., Papadopoulos, I., Skapinakis, P., & Mavreas, V. (2015). Parameters of glucose and lipid metabolism at the fasted state in drug-naïve first-episode patients with psychosis: Evidence for insulin resistance. Psychiatry Research, 229(3), 901–904.
Pillinger, T., Beck, K., Gobjila, C., Donocik, J. G., Jauhar, S., & Howes, O. D. (2017). Impaired glucose homeostasis in first-episode schizophrenia: A systematic review and meta-analysis. JAMA Psychiatry, 74(3), 261–269.
Pringsheim, T., Panagiotopoulos, C., Davidson, J., Ho, J. & Canadian Alliance for Monitoring Effectiveness and Safety of Antipsychotics in Children (CAMESA) guideline group. (2011). Evidence-based recommendations for monitoring safety of second-generation antipsychotics in children and youth. Paediatrics and Child Health, 16(9), 581–589.
Saddichha, S., Manjunatha, N., Ameen, S., & Akhtar, S. (2008). Metabolic syndrome in first episode schizophrenia: A randomized double-blind controlled, short-term prospective study. Schizophrenia Research, 101(1–3), 266–272.
Schneiderhan, M. E., Batscha, C. L., & Rosen, C. (2009). Assessment of a point-of-care metabolic risk screening program in outpatients receiving antipsychotic agents. Pharmacotherapy, 29(8), 975–987.
Thompson, A., Hetrick, S. E., Alvarez-Jiménez, M., Parker, A. G., Willet, M., Hughes, F., … McGorry, P. D. (2011). Targeted intervention to improve monitoring of antipsychotic-induced weight gain and metabolic disturbance in first episode psychosis. Australian and New Zealand Journal of Psychiatry, 45(9), 740–748.
Woo, V., Harris, S. B., & Houlden, R. L. (2005). Canadian Diabetes Association position paper: Antipsychotic medications and associated risks of weight gain and diabetes. Canadian Journal of Diabetes, 29, 111–112.
Author information
Authors and Affiliations
Contributions
All authors have contributed significantly to and agree with content of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no industrial links, affiliations, financial grants, or other sources of funding to disclose.
Additional information
At the time of writing, Dr. Bozymski was a PGY1 Pharmacy Practice Resident at Eskenazi Health in Indianapolis, IN.
Rights and permissions
About this article
Cite this article
Bozymski, K.M., Whitten, J.A., Blair, M.E. et al. Monitoring and Treating Metabolic Abnormalities in Patients with Early Psychosis Initiated on Antipsychotic Medications. Community Ment Health J 54, 717–724 (2018). https://doi.org/10.1007/s10597-017-0203-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10597-017-0203-y