A methodology was developed for the synthesis of 1-ethoxy-6-methyl-2H-[1,2]oxazino[3,4-c]quinoline-2,5(6H)-diones from methyl 4-[2-(dimethylamino)phenyl]-5-ethoxy-6-oxo-6H-1,2-oxazine-3-carboxylates by the action of titanium tetrachloride under inert atmosphere. This interaction provides a rare example of demethylation followed by cyclization, yielding several new compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Nitrogen-containing heterocycles are compounds of key importance for medicinal chemistry. Of particular significance among them are quinolinones,1 widely represented among antibiotics,2,3 psychoactive drugs,4,5,6 anti-inflammatory7,8 and antitumor9 medicines, as well as many other biologically active molecules10,11,12,13,14 (Fig. 1). Therefore, the development of new synthetic routes providing access to compounds of this class remains a valuable goal for organic chemistry.
We have previously found that the treatment of some 2-dimethylaminoarylideneazolones (in particular, benzylidenethiazolidine-2,4-dione) with TiCl4 led to their demethylation, followed by cyclization with the formation of a quinolone ring (Scheme 1).15 At the same time, the [1,5]-hydride shift reaction with subsequent cyclization, which would be the most common outcome for compounds of this type,16,17 did not occur at all. The N-demethylation reaction usually requires specific procedures for tertiary amines – the presence of organometallic catalysts,18,19 enzymes20 or irradiation under certain conditions.21 There are very few literature precedents for N-demethylation reactions followed by cyclization.22

Scheme 1
In the current work, we set out to test the possibility of achieving this type of transformation using compounds derived from our previously obtained 5-ethoxy-6-oxo-1,2-oxazine-3-carboxylates,23,24 if a dimethylamino group could be introduced at position 2 of the phenyl substituent.
The starting 5-ethoxy-6-oxo-1,2-oxazine-3-carboxylate dimethylamino derivatives 1a–f (Scheme 2) were synthesized according to the procedure that we proposed earlier.25,26 As the first step, we synthesized the oxazine ring from the appropriate 2-dimethylaminobenzaldehydes and methyl nitroacetate,24 while in the second step the reaction products were alkylated by the action of triethyloxonium tetrafluoroborate.26 A total of six 5-ethoxy-6-oxo-1,2-oxazine-3-carboxylates 1a–f were obtained, containing a dimethylamino group at the ortho position of the phenyl ring. The obtained compounds were subjected to treatment with TiCl4 in C2H4Cl2 under inert atmosphere. No reaction was observed at room temperature, but heating of the reaction mixture at 85°C for 24 h resulted in the consumption of the starting material and led to the formation of a single product. According to 1H and 13C NMR spectral data, the product in all cases contained only one methyl group at nitrogen atom, while lacking a methyl ester moiety. From these facts it follows that the reaction resulted in the expected demethylation followed by quinolone ring closure. The possible products of [1,5]-hydride shift reaction were also absent in this case. The use of other Lewis acids (SnCl4, AlCl3, BF3·Et2O, Sc(OTf)3) and solvents (PhH, PhMe, CH2Cl2) did not yield the demethylation product, indicating the importance of TiCl4–C2H4Cl2 system for achieving the demethylation reaction.

Scheme 2
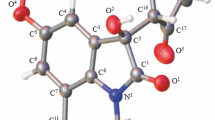
All of the obtained 2H-[1,2]oxazino[3,4-c]quinoline-2,5(6H)-diones 2a–f were characterized by 1H and 13C NMR spectroscopy, as well as by high-resolution mass spectrometry. In the case of compound 2a, the structure was additionally confirmed by two-dimensional NMR experiments – 1H–13C HSQC and 1H–13C HMBC (Fig. 2).
We propose that during the demethylation reaction of 2-(dimethylamino)phenyl-5-ethoxy-6-oxo-1,2-oxazine-3-carboxylates 1a–f TiCl4 interacted with the dimethylamino group, leading to the formation of a stable titanium amide species.27,28 This step was followed by intramolecular reaction of the titanium amide intermediate with carboxy group, resulting in quinoline ring closure (Scheme 3). The absence of [1,5]-hydride shift, in our opinion, can be attributed to the significantly hindered coordination of titanium atom with the carbonyl oxygen atom, when compared to the analogous imidazolone and malonic derivatives.

Scheme 3
Thus, we have developed a new method for the synthesis of previously unknown [1,2]oxazino[3,4-c]-quinoline-2,5(6H)-diones as a result of an unusual demethylation reaction of 2-(dimethylamino)phenyl-5-ethoxy-6-oxo-6H-1,2-oxazine-3-carboxylates that, in turn, can be obtained from 2-dimethylaminobenzaldehydes and methyl nitroacetate. All products were isolated in moderate yields and can be considered as potentially biologically active compounds.
Experimental
1H and 13C NMR spectra were acquired on Bruker Avance III 800 equipped with a cryoprobe (800 and 201 MHz, respectively), Bruker Avance III 700 (700 and 176 MHz, respectively), and Bruker Fourier 300 (300 and 75 MHz, respectively) instruments for samples in DMSO-d6 solutions, using TMS or residual solvent signal as internal standards (2.50 ppm for 1H nuclei, 39.5 ppm for 13C nuclei). High-resolution mass spectra were recorded on an AB Sciex TripleTOF 5600+ instrument in electrospray ionization mode. Melting points were determined on an SMP 30 melting point apparatus. Chromatographic separations were performed on Merck Kieselgel 60 silica gel.
Reagents were purchased from Acros Organics and used without additional purification, while solvents were freshly distilled. Compounds 1a–f were synthesized from substituted 2-dimethylaminobenzaldehydes obtained during our previous work.15
Synthesis of compounds 1a–f (General method). Methyl nitroacetate (785 mg, 6.6 mmol) was added to a solution of the appropriate 2-dimethylaminobenzaldehyde (3 mmol) and diethylamine (548 mg, 7.5 mmol) in MeOH (10 ml). The obtained solution was stirred for 7 days, concentrated to 2–3 ml volume by evaporation, diluted with Et2O (20 ml), and the obtained mixture was maintained in a refrigerator for 12 h at +2°C. The precipitate was filtered off, dissolved in CH2Cl2 (50 ml), and washed with 1% HCl solution (3×20 ml). The organic layer was dried over anhydrous Na2SO4 and evaporated. The residue was taken up in CH2Cl2 (20 ml). The obtained solution was treated with Et3O·BF4 (684 mg, 3.6 mmol), NEt3 (606 mg, 6 mmol), and stirred for 12 h. The obtained mixture was diluted with EtOAc (100 ml), the solution was washed first with a saturated NaHCO3 solution (1×30 ml) and then with saturated KCl solution (2×30 ml), dried over anhydrous Na2SO4, and evaporated to dryness. The residue was purified by the flash chromatography, using 10:1 hexane–EtOAc as eluent.
Methyl 4-[2-(dimethylamino)phenyl]-5-ethoxy-6-oxo-6H-1,2-oxazine-3-carboxylate (1a). Yield 562 mg (59%), yellow powder, mp 104–106°C. 1H NMR spectrum (700 MHz), δ, ppm (J, Hz): 1.18 (3H, t, J = 7.1, CH3); 2.46 (6H, s, 2CH3); 3.67 (3H, s, OCH3); 4.39 (1H, dq, J = 9.9, J = 7.1, CH2); 4.46 (1H, dq, J = 10.1, J = 7.0, CH2); 7.09–7.15 (2H, m, H Ar); 7.34–7.40 (2H, m, H Ar). 13C NMR spectrum (75 MHz), δ, ppm: 15.4; 43.2; 52.7; 69.1; 118.8; 122.5; 123.4; 125.0; 130.5; 131.2; 146.1; 151.0; 151.4; 160.6; 161.1. Found, m/z: 319.1285 [M+H]+. C16H19N2O5. Calculated, m/z: 319.1288.
Methyl 4-[4-chloro-2-(dimethylamino)phenyl]-5-ethoxy-6-oxo-6H-1,2-oxazine-3-carboxylate (1b). Yield 579 mg (55%), yellow powder, mp 108–110°C. 1H NMR spectrum (700 MHz), δ, ppm (J, Hz): 1.19 (3H, t, J = 7.1, CH3); 2.48 (6H, s, 2CH3); 3.71 (3H, s, OCH3); 4.42 (1H, dq, J = 10.0, J = 7.1, CH2); 4.50 (1H, dq, J = 10.0, J = 7.1, CH2); 7.12 (1H, d, J = 2.1, H Ar); 7.17 (1H, dd, J = 8.4, J = 2.1, H Ar); 7.36 (1H, d, J = 8.4, H Ar). 13C NMR spectrum (176 MHz), δ, ppm: 15.3; 42.8; 52.8; 69.2; 118.9; 121.9; 122.1; 123.5; 132.6; 134.7; 146.2; 150.9; 152.5; 160.6; 160.8. Found, m/z: 353.0893 [M+H]+. C16H18ClN2O5. Calculated, m/z: 353.0899.
Methyl 4-[5-chloro-2-(dimethylamino)phenyl]-5-ethoxy-6-oxo-6H-1,2-oxazine-3-carboxylate (1c). Yield 495 mg (47%), yellow powder, mp 150–152°C. 1H NMR spectrum (700 MHz), δ, ppm (J, Hz): 1.20 (3H, t, J = 7.1, CH3); 2.46 (6H, s, 2CH3); 3.70 (3H, s, OCH3); 4.46 (1H, dq, J = 10.1, J = 7.1, CH2); 4.53 (1H, dq, J = 9.9, J = 7.1, CH2); 7.12 (1H, d, J = 8.6, H Ar), 7.40 (1H, d, J = 2.5, H Ar); 7.40–7.44 (1H, m, H Ar). 13C NMR spectrum (75 MHz), δ, ppm: 15.4; 43.1; 52.9; 69.5; 120.7; 122.8; 125.1; 126.1; 130.0; 130.6; 146.5; 150.1; 150.8; 160.6; 160.9. Found, m/z: 353.0895 [M+H]+. C16H18ClN2O5. Calculated, m/z: 353.0899.
Methyl 4-[4-bromo-2-(dimethylamino)phenyl]-5-ethoxy-6-oxo-6H-1,2-oxazine-3-carboxylate (1d). Yield 630 mg (53%), yellow powder, mp 139–141°C. 1H NMR spectrum (700 MHz), δ, ppm (J, Hz): 1.19 (3H, t, J = 7.1, CH3); 2.48 (6H, s, 2CH3); 3.71 (3H, s, OCH3); 4.42 (1H, dq, J = 10.1, J = 7.1, CH2); 4.49 (1H, dq, J = 10.1, J = 7.1, CH2); 7.24 (1H, d, J = 1.7, H Ar); 7.27–7.33 (2H, m, H Ar). 13C NMR spectrum (75 MHz), δ, ppm: 15.4; 42.9; 52.9; 69.3; 121.9; 122.4; 123.4; 123.5; 125.2; 132.9; 146.3; 150.8; 152.6; 160.7; 160.9. Found, m/z: 397.0391 [M+H]+. C16H18BrN2O5. Calculated, m/z: 397.0394.
Methyl 4-[5-bromo-2-(dimethylamino)phenyl]-5-ethoxy-6-oxo-6H-1,2-oxazine-3-carboxylate (1e). Yield 727 mg (61%), yellow powder, mp 153–155°C. 1H NMR spectrum (700 MHz), δ, ppm (J, Hz): 1.21 (3H, t, J = 7.1, CH3); 2.46 (6H, s, 2CH3); 3.70 (3H, s, OCH3); 4.45 (1H, dq, J = 10.1, J = 7.1, CH2); 4.54 (1H, dq, J = 10.1, J = 7.1, CH2); 7.06 (1H, d, J = 8.6, H Ar); 7.51–7.55 (2H, m, H Ar). 13C NMR spectrum (176 MHz), δ, ppm: 15.3; 42.9; 52.7; 69.4; 113.8; 120.9; 122.8; 125.3; 132.8; 133.3; 146.4; 150.5; 150.7; 160.5; 160.8. Found, m/z: 397.0388 [M+H]+. C16H18BrN2O5. Calculated, m/z: 397.0394.
Methyl 4-[2-(dimethylamino)-4-methoxyphenyl]-5-ethoxy-6-oxo-6H-1,2-oxazine-3-carboxylate (1f). Yield 677 mg (65%), yellow powder, mp 102–104°C. 1H NMR spectrum (700 MHz), δ, ppm (J, Hz): 1.19 (3H, t, J = 7.1, CH3); 2.46 (6H, s, 2CH3); 3.69 (3H, s, OCH3); 3.80 (3H, s, OCH3); 4.36 (1H, dq, J = 10.1, J = 7.0, CH2); 4.42 (1H, dq, J = 9.9, J = 7.0, CH2); 6.60 (1H, d, J = 2.5, H Ar); 6.70 (1H, dd, J = 8.5, J = 2.4, H Ar); 7.29 (1H, d, J = 8.6, H Ar). 13C NMR spectrum (176 MHz), δ, ppm: 15.3; 42.9; 52.6; 55.1; 68.8; 104.8; 107.6; 115.2; 125.1; 132.3; 145.5; 151.6; 152.6; 160.6; 161.0; 161.1. Found, m/z: 349.1391 [M+H]+. C17H21BrN2O6. Calculated, m/z: 349.1394.
Synthesis of compounds 2a–f (General method). A stirred solution of the appropriate compound 1a–f (0.5 mmol) in anhydrous C2H4Cl2 (5 ml) was treated under argon atmosphere by dropwise addition of TiCl4 (143 mg, 0.75 mmol) solution in anhydrous C2H4Cl2 (1 ml) over 5 min. The obtained solution was stirred at 85°C for 24 h. The reaction mixture was cooled to 25°C, quenched with 3% NaHCO3 solution (50 ml), and the obtained mixture was extracted with EtOAc (3×50 ml). The combined organic layers were washed with a saturated NaCl solution (3×50 ml), dried over anhydrous Na2SO4, and evaporated to dryness. The residue was purified by column chromatography, using EtOAc as eluent.
1-Ethoxy-6-methyl-2H-[1,2]oxazino[3,4-c]quinoline-2,5(6H)-dione (2a). Yield 65 mg (48%), yellow powder, mp 187–189°C. 1H NMR spectrum (700 MHz, CDCl3), δ, ppm (J, Hz): 1.56 (3H, t, J = 7.3, CH3); 3.68 (3H, s, CH3); 4.89 (2H, q, J = 7.1, CH2); 7.28–7.31 (2H, m, H Ar); 7.60 (1H, td, J = 7.9, J = 1.4, H Ar); 8.92 (1H, dd, J = 8.6, J = 1.5, H Ar). 13C NMR spectrum (176 MHz, CDCl3), δ, ppm: 16.0; 30.6; 71.0; 114.8; 115.3; 117.7; 123.9; 130.5; 132.5; 138.0; 144.3; 145.2; 155.6; 161.4. Found, m/z: 273.0871 [M+H]+. C14H13N2O4. Calculated, m/z: 273.0870.
8-Chloro-1-ethoxy-6-methyl-2H-[1,2]oxazino[3,4-c]-quinoline-2,5(6H)-dione (2b). Yield 63 mg (41%), brown powder, mp 215–217°C. 1H NMR spectrum (700 MHz), δ, ppm (J, Hz): 1.44 (3H, t, J = 7.1, CH3); 3.55 (3H, s, CH3); 4.72 (2H, q, J = 7.1, CH2); 7.40 (1H, dd, J = 8.8, J = 2.1, H Ar); 7.53 (1H, d, J = 2.1, H Ar); 8.81 (1H, d, J = 8.8, H Ar). 13C NMR spectrum (201 MHz), δ, ppm: 15.7; 30.3; 70.4; 113.4; 115.8; 123.2; 130.9; 136.6; 139.2; 144.3; 145.2; 154.9; 161.3. Found, m/z: 307.0483 [M+H]+. C14H12ClN2O4. Calculated, m/z: 307.0480.
9-Chloro-1-ethoxy-6-methyl-2H-[1,2]oxazino[3,4-c]-quinoline-2,5(6H)-dione (2c). Yield 60 mg (39%), brown powder, mp 173–175°C. 1H NMR spectrum (700 MHz), δ, ppm (J, Hz): 1.47 (3H, t, J = 7.1, CH3); 3.54 (3H, s, CH3); 4.78 (2H, q, J = 7.1, CH2); 7.49 (1H, d, J = 9.2, H Ar); 7.70 (1H, dd, J = 9.0, J = 2.3, H Ar); 8.82 (1H, d, J = 2.5, H Ar). 13C NMR spectrum (201 MHz), δ, ppm: 15.7; 30.3; 70.8; 116.0; 116.5; 117.8; 127.2; 128.4; 131.5; 136.8; 144.9; 145.1; 154.7; 161.2. Found, m/z: 307.0483 [M+H]+. C14H12ClN2O4. Calculated, m/z: 307.0480.
8-Bromo-1-ethoxy-6-methyl-2H-[1,2]oxazino[3,4-c]-quinoline-2,5(6H)-dione (2d). Yield 74 mg (43%), yellow powder, mp 199–201°C. 1H NMR spectrum (700 MHz), δ, ppm (J, Hz): 1.44 (3H, t, J = 7.1, CH3); 3.54 (3H, s, CH3); 4.72 (2H, q, J = 7.1, CH2); 7.53 (1H, dd, J = 8.9, J = 2.0, H Ar); 7.64 (1H, d, J = 1.9, H Ar); 8.72 (1H, d, J = 8.8, H Ar). 13C NMR spectrum (75 MHz), δ, ppm: 15.8; 30.4; 70.5; 113.8; 117.5; 118.7; 125.5; 126.3; 131.0; 139.2; 144.5; 145.2; 155.0; 161.4. Found, m/z: 350.9979 [M+H]+. C14H12BrN2O4. Calculated, m/z: 350.9975.
9-Bromo-1-ethoxy-6-methyl-2H-[1,2]oxazino[3,4-c]-quinoline-2,5(6H)-dione (2e). Yield 82 mg (47%), yellow powder, mp 203–208°C. 1H NMR spectrum (700 MHz), δ, ppm (J, Hz): 1.47 (3H, t, J = 7.1, CH3); 3.53 (3H, s, CH3); 4.77 (2H, q, J = 7.1, CH2); 7.43 (1H, d, J = 9.0, H Ar); 7.81 (1H, dd, J = 9.0, J = 2.3, H Ar); 8.97 (1H, d, J = 2.3, H Ar). 13C NMR spectrum (201 MHz), δ, ppm: 15.6; 30.3; 70.8; 115.1; 116.3; 118.1; 131.3; 134.4; 137.1; 144.9; 145.1; 154.7; 161.1. Found, m/z: 350.9976 [M+H]+. C14H12BrN2O4. Calculated, m/z: 350.9975.
1-Ethoxy-8-methoxy-6-methyl-2H-[1,2]oxazino[3,4-c]-quinoline-2,5(6H)-dione (2f). Yield 75 mg (50%), white powder, mp 201–203°C. 1H NMR spectrum (700 MHz), δ, ppm (J, Hz): 1.42 (3H, t, J = 7.1, CH3); 3.54 (3H, s, CH3); 3.92 (3H, s, OCH3); 4.63 (2H, q, J = 7.1, CH2); 6.92 (1H, d, J = 2.7, H Ar); 6.96 (1H, dd, J = 9.1, J = 2.6, H Ar); 8.79 (1H, d, J = 9.2, H Ar). 13C NMR spectrum (176 MHz), δ, ppm: 15.6; 30.1; 55.7; 69.6; 101.8; 107.1; 109.1; 118.7; 131.5; 139.8; 142.0; 145.2; 155.0; 161.5; 162.2. Found, m/z: 303.0978 [M+H]+. C15H15N2O5. Calculated, m/z: 303.0975.
Supplementary information file containing 1H, 13C NMR and high-resolution mass spectra of all synthesized compounds, as well as two-dimensional 1H–13C HSQC and 1H–13C HMBC spectra of compound 2a is available at the journal website http://springerlink.bibliotecabuap.elogim.com/journal/10593.
References
Shiro, T.; Fukaya, T.; Tobe, M. Eur. J. Med. Chem. 2015, 97, 397.
Manvar, A.; Bavishi, A.; Radadiya, A.; Patel, J.; Vora, V.; Dodia, N.; Rawal, K.; Shah, A. Bioorg. Med. Chem. Lett. 2011, 21, 4728.
Haemers, A.; Leysen, D. C.; Bollaert, W.; Zhang, M. Q.; Pattyn, S. R. Antimicrob. Agents Chemother. 1990, 34, 496.
Thomas, R. L.; Langmead, C. J.; Wood, M. D.; Challiss, R. A. J. J. Pharmacol. Exp. Ther. 2009, 331, 1086.
Gibbons, A.; Dean, B. Curr. Pharm. Des. 2016, 22, 2124.
Frampton, J. E. Drugs 2017, 77, 2049.
Coskun, M.; Vermeire, S.; Nielsen, O. H. Trends Pharmacol. Sci. 2017, 38, 127.
Olivera, P.; Danese, S.; Peyrin-Biroulet, L. Gut 2017, 66, 199.
Brnardic, E. J.; Garbaccio, R. M.; Fraley, M. E.; Tasber, E. S.; Steen, J. T.; Arrington, K. L.; Dudkin, V. Y.; Hartman, G. D.; Stirdivant, S. M.; Drakas, B. A.; Rickert, K.; Walsh, E. S.; Hamilton, K.; Buser, C. A.; Hardwick, J.; Tao, W.; Beck, S. C.; Mao, X.; Lobell, R. B.; Sepp-Lorenzino, L.; Yan, Y.; Ikuta, M.; Munshi, S. K.; Kuo, L. C.; Kreatsoulas, C. Bioorg. Med. Chem. Lett. 2007, 17, 5989.
Sun, Y.; Han, J.; Wang, Z.; Li, X.; Sun, Y.; Hu, Z. Front. Pharmacol. 2021, 11, 621093. DOI https://doi.org/10.3389/fphar.2020.621093.
Staun-Ram, E.; Miller, A. Clin. Immunol. 2017, 184, 11.
Kumar, S.; Singh, R. K.; Patial, B.; Goyal, S.; Bhardwaj, T. R. J. Enzyme Inhib. Med. Chem. 2016, 31, 173.
Norman, B. H.; Lander, P. A.; Gruber, J. M.; Kroin, J. S.; Cohen, J. D.; Jungheim, L. N.; Starling, J. J.; Law, K. L.; Self, T. D.; Tabas, L. B.; Williams, D. C.; Paul, D. C.; Dantzig, A. H. Bioorg. Med. Chem. Lett. 2005, 15, 5526.
Potapov, A. Yu.; Paponov, B. V.; Podoplelova, N. A.; Panteleev, M. A.; Polikarchuk, V. A.; Ledenyova, I. V.; Stolpovskaya, N. V.; Kryl'skii, D. V.; Shikhaliev, Kh. S. Russ. Chem. Bull. 2021, 70, 492.
Zaitseva, E. R.; Ivanov, D. S.; Sokolov, A. I.; Mikhaylov, A. A.; Baleeva, N. S.; Korlyukov, A. A.; Myasnyanko, I. N.; Smirnov, A. Y.; Baranov, M. S. ChemistrySelect 2022, 7, e202203294.
Zaitseva, E. R.; Smirnov, A. Yu.; Myasnyanko, I. N.; Mineev, K. S.; Sokolov, A. I.; Volkhina, T. N.; Mikhaylov, A. A.; Baleeva, N. S.; Baranov, M. S. New J. Chem. 2021, 45, 1805.
Zaitseva, E. R.; Smirnov, A. Yu.; Baleeva, N. S.; Baranov, M. S. Chem. Heterocycl. Compd. 2021, 57, 695.
Maiti, D.; Narducci Sarjeant, A. A.; Karlin, K. D. J. Am. Chem. Soc. 2007, 129, 6720.
Comba, P.; Kuwata, S.; Linti, G.; Pritzkow, H.; Tarnai, M.; Wadepohl, H. Chem. Commun. 2006, 2074.
Kedderis, G. L.; Hollenberg, P. F. Arch. Biochem. Biophys. 1984, 233, 315.
Ripper, J. A.; Tiekink, E. R. T.; Scammells, P. J. Bioorg. Med. Chem. Lett. 2001, 11, 443.
Shirokova, V. V.; Ikonnikova, V. A.; Solyev, P. N.; Lushpa, V. A.; Korlyukov, A. A.; Volodin, A. D.; Baleeva, N. S.; Baranov, M. S.; Mikhaylov, A. A. Synthesis 2021, 4689.
Baranov, M. S.; Yampolsky, I. V. Tetrahedron Lett. 2013, 54, 628.
Smirnov, A. Yu.; Zaitseva, E. R.; Belozerova, O. A.; Alekseyev, R. S.; Baleeva, N. S.; Zagudaylova, M. B.; Mikhaylov, A. A.; Baranov, M. S. J. Org. Chem. 2019, 84, 15417.
Zaitseva, E. R.; Smirnov, A. Yu.; Baleeva, N. S.; Baranov, M. S. Chem. Heterocycl. Compd. 2019, 55, 676.
Nizovtsev, A. V; Sokolov, A. I.; Smirnov, A. Yu.; Mikhaylov, A. A.; Myasnyanko, I. N.; Belozerova, O. A.; Baleeva, N. S.; Usmanova, L.; Baranov, M. S. ChemistrySelect 2021, 6, 8938.
Leggio, A.; Comandè, A.; Belsito, E. L.; Greco, M.; Lo Feudo, L.; Liguori, A. Org. Biomol. Chem. 2018, 16, 5677.
Shono, T.; Matsumura, Y.; Inoue, K.; Ohmizu, H.; Kashimura, S. J. Am. Chem. Soc. 1982, 104, 5753.
This study received financial support from the Russian Science Foundation (project No. 20-73-10195).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2023, 59(4/5), 327–330
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zaitseva, E.R., Ivanov, D.S., Smirnov, A.Y. et al. Synthesis of 2H-[1,2]oxazino[3,4-c]quinoline-2,5(6H)-diones from 6-oxo-6H-1,2-oxazine-3-carboxylates. Chem Heterocycl Comp 59, 327–330 (2023). https://doi.org/10.1007/s10593-023-03201-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-023-03201-2