A novel methodology has been developed to afford 1,4,5-trisubstituted 1-benzyl-5-formyl-1,2,3-triazole-4-carboxylates in one step under mild conditions. This method includes the reduction of the ester functional group to an aldehyde using lithium tri-tert-butoxyaluminum hydride. Especially, this method offers a different perspective for fully substituted 1,2,3-triazoles with high regioselectivity and high yields. The structure of fully substituted triazoles was verified using FTIR, 1H, 13C NMR spectroscopy, HRMS, advanced NMR techniques (COSY, C-APT, HSQC, and HMBC), and X-ray crystallography.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1,2,3-Triazoles have been described in the literature as important heterocycles with pharmaceutical properties and biological activities thanks to the development of the cycloaddition reaction by Huisgen.1,2,3,4 These motifs can be encountered widely in organic synthesis, materials science, dye chemistry, and agriculture chemistry.5,6,7 The number of the synthesis designs of 1,2,3-triazoles is increasing day by day owing to their broad application areas. There are different synthetic strategies in the literature for the synthesis of 1,2,3-triazoles, but the most preferred pathway for this type of compounds is based on Sharpless and Fokin's methodologies.8,9,10,11 However, the literature reporting the formation of fully substituted 1,2,3-triazoles appears to be less common compared to the literature related to the formation of 1,4- or 1,5-disubstituted 1,2,3-triazoles. In general, fully substituted triazole derivatives can be obtained in metal-catalyzed and metal-free reactions.12,13,14,15,16,17,18,19,20,21 These reactions are based on the 1,3-cycloaddition reaction, and such reagents as α-bromoacroleins, enaminones, 1,3-dicarbonyl compounds, β-ketosulfones are generally used in these transformations.14 The known studies on 1,4,5-trisubstituted-1,2,3-triazoles stand out as enamine approach and 1,3-dipolar cycloadditions. The regioselective synthesis of asymmetric 1,4,5-trisubstituted 1,2,3-triazoles is not as easy as the regioselective synthesis of 1,4- or 1,5-disubstituted 1,2,3-triazoles, which brings to a major limitation due to unselectivity and difficulty in separation of two isomers. Ramanaiah et al. have shown that the C4/C5 selectivity in the direct addition of unsymmetric alkynes of 1,2,3-triazole is strikingly sensitive to conventional reaction parameters and two isomers undergo the same reaction, however, separation attempts were unsuccessful (Scheme 1a).15 Pokhodylo et al. reported the synthesis of 5-formyl-1,2,3-triazole-4-carboxylate derivatives in the reaction between ethyl 4,4-diethoxy-3-oxobutanoate and N-substituted phenyl azides in high yields (Scheme 1b).16 Although the fully substituted 1,2,3-triazoles were obtained in high yields the scope was limited. Moreover, some ethyl 1-aryl-5-(diethoxy-methlyl)-1H-1,2,3-triazole-4-carboxylate derivatives were converted to aldehydes while some remained stable as geminal diols (Scheme 1b).16 Additionally, this method is not very advantageous since the starting compounds used in this method are often expensive. Most of the strategies to access 1,4,5-trisubstituted 1,2,3-triazoles have constraints such as high cost of metal catalyst, isomer formation, low yield, and difficulties encountered during isolation. In the reaction of internal alkynes substituted with different carbonyl groups (formyl, ester, or monodialkylacetal) with organic azides in the thermal and metal-free conditions, two different regioisomers, N-alkyl-substituted 4-formyl-1,2,3-triazole-5-carboxylates and N-alkyl-substituted 5-formyl-1,2,3-triazole-4-carboxylates have been isolated.15,17 All these reports confirm that the metal catalyst is inevitable to control the regioselectivity in the reactions of unsymmetrical alkynes.15,16,17,18,19,20,21,22

Scheme 1
Aldehydes are among the most reactive functional groups that undergo a wide variety of chemical reactions with nucleophiles resulting in both reversible and irreversible bond formation.23 Generally, lithium tri-tert-butoxy-aluminum hydride (LTBA) is used as a reducing agent to obtain the formyl group from acyl halides and esters. However, it might fail in the reduction of the ester group toward aldehyde in the azoles (Scheme 1c).24,25,26,27,28,29,30 Lopchuk and Gribble reported a two-step synthesis of 2-formylindole, where initially ester was reduced by DIBAL-H followed by oxidation with MnO2 (Scheme 1c).27 In 2021, Deng et al. reported that reduction of an ester group at pyrrole ring resulted in formation of primary alcohol that was further oxidized toward aldehyde using the Ley– Griffith-type method (Scheme 1c).28 Although selective formation of alcohols from azoles containing an ester group can be achieved by using LiAlH4, NaBH4, DIBAL-H, LTBA, it is almost impossible to encounter selective formyl group synthesis in one step.24,25,26,27,28,29,30 Herein we report a method that affords fully substituted 1,2,3-triazoles containing aldehyde and ester groups in one step in excellent yield and high regioselectivity.
In the development of the regioselective synthesis of 1,2,3-triazoles, we envisioned to fulfill such criteria as short reaction time, safe conditions, cost effectiveness, and high-yielding process. Additionally, environmentally benign and metal-free conditions were preferred. In this study, we focused on the one-step synthesis of unsymmetrical 1,4,5-trisubstituted triazoles containing ester and formyl functionalities in positions 4 and 5, respectively. To access these structures, 4,5-diester-1,2,3-triazoles 2a–m were initially synthesized using 1,3-cycloaddition reaction between benzyl azides 1a–m and acetylene dicarboxylate by following the literature (Scheme 2).31,32,33,34,35,36,37,38

Scheme 2
Dimethyl 1-benzyl-1H-1,2,3-triazole-4,5-dicarboxylate (2a) was chosen as a model substrate to develop a selective reduction by using LTBA as a bulky, strong reducing reagent. To determine the optimal conditions, solvents (Et2O or THF), temperature, and amount of the base were screened (Table 1).
Initially diester 2a was treated with 3.0 equiv of LTBA in Et2O at –20°C for 3 h (Table 1, entry 1). Under these conditions, diester 2a was not converted to products 3a or 4a. Increase of temperature to –10°C did not facilitate the reaction (entry 2). We then investigated different solvents. Treatment of diester 2a with LTBA at –78°C in THF also did not produce the target structures (entries 3, 4). When 3.0 equiv of LTBA were used at –35°C, the formation of aldehyde 3a and alcohol 4a was detected, albeit in low yield (entry 6). With the information obtained from entries 1–6 we realized that the temperature should reach some threshold value to facilitate the target reaction.
Reduction of ester 2a in THF at –20°C in the presence of 1.2 equiv of LTBA afforded 10% of aldehyde 3a (entry 7). When the same conditions were applied but the amount of LTBA increased to 1.5 equiv, significant amount of compound 3a was detected together with alcohol 4a (entry 8). Further increase of temperature to –10°C provided a significant amount of products 3a/4a compared to entries 1–8 (entry 9). Even though the increase in temperature and amount of LTBA provided the desired aldehyde 3a, these conditions also facilitated the formation of alcohol 4a and caused significant yield loss. The highest yield of aldehyde 3a was achieved at –10 to –5°C with 1.8 equiv of LTBA (entry 10). Even if the amount of LTBA was significantly increased, no reduction at position 4 of the triazole ring was observed. This gave us a chance to access the structural control of our targets.
With the optimized conditions in hand, 13 different fully substituted 1,2,3-triazoles 3a–m were synthesized together with alcohols 4a–m (Scheme 3). Aldehydes containing F, Cl, OMe, and NO2 group were obtained in high yields. The integrity of the 1,4,5-trisubstituted 1,2,3-triazole derivatives was validated by 1H, 13C NMR, 2D NMR techniques, and X-ray crystallography.

Scheme 3
IR analysis of diesters 2a–m revealed strong bands at 1730–1725 cm–1 which corresponds to the ester functional groups. Furthermore, the methoxy group protons of compounds 2a–m resonate at around 4.10 and 3.80 ppm in the 1H NMR spectra. Additionally, quaternary carbons C-4 and C-5 of the 1,2,3-triazole ring appear at around 130–145 ppm in 13C NMR spectra, and ester carbonyl groups give two different C signals in the range of 158–165 ppm.
To fully confirm structures of aldehydes 3a–m and alcohols 4a–m, 2D NMR experiments (1H–13C HSQC and 1H–13C HMBC) were performed for the representative compounds 4a and 3l. In the 1H NMR spectrum of compound 4a, signals of 13 protons with seven different chemical shifts were obtained. 10 Carbon atoms, 3 of which are in the aliphatic region, resonate in the 13C NMR spectrum. Firstly, the locations of the carbon atoms that resonate in positive and negative amplitudes were determined by 13C-APT NMR technique. In this information, CH and CH3 carbons are assigned to the signals at 52.5, 127.4, 128.8, and 129.2 ppm; it was also found that signals at 162.8, 52.7, 53.7, 134.0, 137.1, and 141.0 ppm belong to CH2 and quaternary carbon atoms. After this initial assignment, correlations between proton and carbon signals were determined using the 1H–13C HSQC experiment (Fig. 1) as reported in Table 2.
In the 1H–13C HMBC spectrum of compound 4a (Fig. 2), quaternary carbon C-11 resonating at 134.0 ppm gives correlation with CH2 group through 2 bonds. Additional correlations with H-14, H-13, and H-12 protons on the benzene ring were also observed through 2–4 bonds. Thus, it was determined that the C-11 carbon corresponds to the benzene ring. The positions of the other 2 quaternary carbon atoms were also examined taking into account correlations of the carbon at 137.1 ppm. It can be clearly seen that it interacts less with the benzylic CH2 hydrogens while it intensely interacts with the 6-CH2 together with little interaction with the 9-CH3 protons as well. It has been determined that the carbon atom at 141.0 ppm interacts intensely with both H-12 and 6-OH, but does not interact with methoxy 9-CH3. When all these spectral data are interpreted together carbon atoms C-4 and C-5 were assigned to the triazole ring. According to the 2D NMR results, the ester functional group is attached to the C-4 carbon and the one at the C-5 carbon is reduced to a hydroxymethyl group.
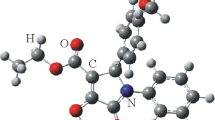
A tentative approach to the formation of compound 3a is outlined in Scheme 4. Although theoretically two products 3a and 3a' are possible, only aldehyde 3a was isolated. The structure of compound 3a was supported with 1H–13C HBMC spectra. The bulky nature of LTBA may have led to product 3a instead 3a'. However, it should also be noted that the bulkiness of the C-5 and N-substituted benzyl groups will probably cause additional instability in the product, and thus, a more stable product formation will be observed upon reduction at the C-5 position. In a similar study by Butler et al. in 2021, compound 3a was used as the substrate and only the C-5 position was reduced after treatment with NaBH4.39 Similar to our study, the amount of NaBH4 did not show any impact on regioisomer formation ratios. Thus, electron density differences are postulated to explain the faster rates of reduction at the C-5 esters. In addition, Yasui et al. reported that one of the ester groups in 2 different positions of the pyrrole ring was selectively reduced. They put forward that this chemoselectivity is due to the chelate formation of aluminum with the unshared electron pairs on the carbonyl oxygen with the nitrogen on the pyrrole ring, and so the non-chelating ester group was selectively reduced.30 Analogously, it can be thought that in our study, the ester group at position 4 and the N atom at position 3 can form chelate with aluminum and so only the C-5 position can be reduced. Besides all these discussions, X-ray data confirmed the structures of compounds 3g,l unambiguously (Figs. 3 and 4).

Scheme 4
To sum up, we developed a new and low-cost regioselective method to access a 1,4,5-trisubstitued 1,2,3-triazoles. Our strategy offers the one-step method to afford aldehydes from the esters in the azoles by using bulky lithium tri-tert-butoxyaluminum hydride. The metal-free nature of this method may be preferable to methods that require metal catalysis, especially for biological studies. We anticipate that our strategy will find extensive application in the synthesis of fully substituted 1,2,3-triazoles containing the formyl functional group.
Experimental
IR spectra were recorded as neat using a Bruker ATR-FT-IR spectrometer. 1H and 13C spectra were recorded on a Bruker spectrometer (300 and 75 MHz, respectively) in CDCl3 with TMS as internal standard. High-resolution mass spectrometry data were acquired on an Agilent Technologies 6224 TOF LC/MS. Melting points were recorded with a STUART SMP-30 device. The reactions progress was monitored by thin-layer chromatography (TLC) using Merck silica gel 60 F254 aluminum plates with 0.25-mm thickness. The aluminum plates were visualized by an ultraviolet lamp (254 nm). Column chromatography was carried out using Merck silica gel 60 (0.040–0.063 mm).
All the reagents used in the synthesis were purchased from commercial suppliers Sigma-Aldrich and Acros. For purification, the solvents of analytical grade were used or were distilled.
Synthesis of 1,2,3-triazoles 2a–m (General method). A mixture of substituted benzyl azide 1a–m (1.0 mmol) and dimethyl but-2-ynedioate (1.0 mmol) was heated at 100°C for 5 min without any solvent or metal.31,32,33,34,35,36,37,38 After 5 min (monitored by TLC), the reaction mixture was cooled to room temperature and purified by column chromatography on silica gel using elution with hexane–EtOAc, 3:2 (for compounds 2a–j) or 1:1(for compounds 2k–m).
Dimethyl 1-benzyl-1H-1,2,3-triazole-4,5-dicarboxylate (2a).31 Yield 270 mg (98%), white solid, mp 48–49°C. IR spectrum, ν, cm–1: 2958, 1723, 1491, 1435. 1H NMR spectrum, δ, ppm: 7.32–7.25 (5H, m, H Ar); 5.80 (2H, s, CH2); 3.95 (3H, s, OCH3); 3.87 (3H, s, OCH3). 13C NMR spectrum, δ, ppm: 160.4; 158.8; 140.2; 133.9; 129.8; 128.8 (2C); 128.2; 53.9; 53.3; 52.7. Found, m/z: 298.0790 [M+Na]+. C13H13N3NaO4. Calculated, m/z: 298.0798.
Dimethyl 1-[(2-fluorophenyl)methyl]-1H-1,2,3-triazole-4,5-dicarboxylate (2b).35 Yield 269 mg (92%), white solid, mp 71–72°C. IR spectrum, ν, cm–1: 3074, 2954, 1741, 1720, 1618, 1461. 1H NMR spectrum, δ, ppm: 7.34–7.11 (4H, m, H Ar); 5.86 (2H, s, CH2); 3.96 (3H, s, OCH3); 3.92 (3H, s, OCH3). 13C NMR spectrum, δ, ppm (J, Hz): 159.5; 159.4 (d, J = 248.9); 157.8; 139.1; 130.1 (d, J = 8.2); 129.5; 129.0 (d, J = 3.3); 123.9 (d, J = 3.8); 120.5 (d, J = 14.3); 114.9 (d, J = 21.1); 52.6; 51.9; 46.8. Found, m/z: 316.0688 [M+Na]+. C13H12FN3NaO4. Calculated, m/z: 316.0704.
Dimethyl 1-[(3-fluorophenyl)methyl]-1H-1,2,3-triazole-4,5-dicarboxylate (2c).38 Yield 260 mg (89%), yellow liquid. IR spectrum, ν, cm–1: 2958, 1727, 1611, 1519. 1H NMR spectrum, δ, ppm: 7.37–7.24 (2H, m, H Ar); 7.08–6.94 (2H, m, H Ar); 5.81 (2H, s, CH2); 3.97 (3H, s, OCH3); 3.91 (3H, s, OCH3). 13C NMR spectrum, δ, ppm (J, Hz): 161.9 (d, J = 247.9); 159.5; 157.8; 139.6; 135.3 (d, J = 7.5); 129.7 (d, J = 8.2); 128.7; 122.8; 115.0 (d, J = 21.0); 114.2 (d, J = 22.5); 52.5; 52.4; 51.9. Found, m/z: 316.0696 [M+Na]+. C13H12FN3NaO4. Calculated, m/z: 316.0704.
Dimethyl 1-[(4-fluorophenyl)methyl]-1H-1,2,3-triazole-4,5-dicarboxylate (2d).35 Yield 253 mg (86%,) white solid, mp 109–110°C. IR spectrum, ν, cm–1: 2955, 1723, 1606, 1419. 1H NMR spectrum, δ, ppm (J, Hz): 7.28 (2H, d, AA'XX' system, J = 13.8, H Ar); 7.03 (2H, d, AA'XX' system, J = 13.8, H Ar); 5.78 (2H, s, CH2); 3.96 (3H, s, OCH3); 3.90 (3H, s, OCH3). 13C NMR spectrum, δ, ppm (J, Hz): 162.8 (d, J = 248.6); 160.4; 158.8; 140.3; 130.1 (d, J = 8.5); 129.8; 129.4; 115.9 (d, J = 22.0); 53.3; 53.1; 52.7. Found, m/z: 316.0693 [M+Na]+. C13H12FN3NaO4. Calculated, m/z: 316.0704.
Dimethyl 1-[(2-chlorophenyl)methyl]-1H-1,2,3-triazole-4,5-dicarboxylate (2e).35 Yield 280 mg (91%), white solid, mp 95–96°C. IR spectrum, ν, cm–1: 3009, 2954, 1727, 1581, 1468. 1H NMR spectrum, δ, ppm (J, Hz): 7.42 (1H, d, J = 7.9, H Ar); 7.33–7.15 (2H, m, H Ar); 6.88 (1H, d, J = 7.9, H Ar); 5.93 (2H, s, CH2); 3.98 (3H, s, OCH3); 3.89 (3H, s, OCH3). 13C NMR spectrum, δ, ppm: 160.3; 158.6; 140.0; 132.9; 131.9; 130.6; 129.9; 129.8; 128.8; 127.3; 53.4; 52.7; 51.3. Found, m/z: 310.0581 [M+H]+. C13H13ClN3O4. Calculated, m/z: 310.0585.
Dimethyl 1-[(3-chlorophenyl)methyl]-1H-1,2,3-triazole-4,5-dicarboxylate (2f).37 Yield 293 mg (95%), yellow liquid. IR spectrum, ν, cm–1: 2964, 1729, 1662, 1588, 1464. 1H NMR spectrum, δ, ppm (J, Hz): 7.29–7.27 (4H, m, H Ar); 7.15 (1H, d, J = 4.8, H Ar); 5.78 (2H, s, CH2); 3.96 (3H, s, OCH3); 3.91 (3H, s, OCH3). 13C NMR spectrum, δ, ppm: 160.4; 158.6; 140.5; 135.7; 134.7; 130.3; 129.3; 129.1; 128.3; 126.1; 53.4; 53.2; 52.8. Found, m/z: 332.0396 [M+Na]+. C13H12ClN3NaO4. Calculated, m/z: 332.0409.
Dimethyl 1-[(4-chlorophenyl)methyl]-1H-1,2,3-triazole-4,5-dicarboxylate (2g).35 Yield 300 mg (97%), white solid, mp 83–84°C. IR spectrum, ν, cm–1: 3097, 2952, 1725, 1547. 1H NMR spectrum, δ, ppm (J, Hz): 7.31 (2H, d, AA'XX' system, J = 8.5, H Ar); 7.23 (2H, d, AA'XX' system, J = 8.5, H Ar); 5.78 (2H, s, CH2); 3.96 (3H, s, OCH3); 3.91 (3H, s, OCH3). 13C NMR spectrum, δ, ppm: 160.4; 158.7; 140.7; 134.8; 132.3; 129.6; 129.1; 53.4; 53.0; 52.8. Found, m/z: 310.0599 [M+H]+. C13H13ClN3O4. Calculated, m/z: 310.0594.
Dimethyl 1-[(2-methoxyphenyl)methyl]-1H-1,2,3-triazole-4,5-dicarboxylate (2h). Yield 302 mg (99%), white solid, mp 99–100°C. IR spectrum, ν, cm–1: 2959, 1731, 1602, 1490. 1H NMR spectrum, δ, ppm: 7.16–6.84 (4H, m, H Ar); 5.76 (2H, s, CH2); 3.95 (3H, s, OCH3); 3.90 (3H, s, OCH3); 3.76 (3H, s, OCH3). 13C NMR spectrum, δ, ppm: 160.4; 158.6; 157.1; 138.8; 130.8; 130.4; 129.7; 122.1; 120.6; 110.6; 55.3; 53.2; 52.5; 49.5. Found, m/z: 328.0893 [M+Na]+. C14H15N3NaO5. Calculated, m/z: 328.0904.
Dimethyl 1-[(3-methoxyphenyl)methyl]-1H-1,2,3-triazole-4,5-dicarboxylate (2i). Yield 284 mg (93%), yellow liquid. IR spectrum, ν, cm–1: 2958, 1731, 1602, 1490. 1H NMR spectrum, δ, ppm: 7.26–6.79 (4H, m, H Ar); 5.78 (2H, s, CH2); 3.97 (3H, s, OCH3); 3.90 (3H, s, OCH3); 3.78 (3H, s, OCH3). 13C NMR spectrum, δ, ppm: 159.5; 159.0; 158.6; 139.1; 134.4; 129.1; 119.2; 113.4; 112.6; 54.3; 52.9; 52.3; 51.7. Found, m/z: 328.0901 [M+Na]+. C14H15N3NaO5. Calculated, m/z: 328.0904.
Dimethyl 1-[(4-methoxyphenyl)methyl]-1H-1,2,3-triazole-4,5-dicarboxylate (2j).36 Yield 286 mg (94%), white solid, mp 70–71°C. IR spectrum, ν, cm–1: 2958, 1730, 1578, 1552. 1H NMR spectrum, δ, ppm (J, Hz): 7.23 (2H, d, AA'XX' system, J = 8.6, H Ar); 6.85 (2H, d, AA'XX' system, J = 8.7, H Ar); 5.73 (2H, s, CH2); 3.95 (3H, s, OCH3); 3.89 (3H, s, OCH3); 3.78 (3H, s, OCH3). 13C NMR spectrum, δ, ppm: 160.4; 160.0; 159.2; 140.2; 130.0; 125.9 (2C); 114.5; 54.9; 53.5; 53.3; 52.5. Found, m/z: 306.1085 [M+H]+. C14H16N3O5. Calculated, m/z: 306.1085.
Dimethyl 1-[(2-nitrophenyl)methyl]-1H-1,2,3-triazole-4,5-dicarboxylate (2k).36 Yield 262 mg (82%), white solid, mp 90–92°C. IR spectrum, ν, cm–1: 2960, 1731, 1552, 1519. 1H NMR spectrum, δ, ppm (J, Hz): 8.17 (1H, d, J = 8.1, H Ar); 7.63–7.47 (2H, m, H Ar); 6.86 (1H, d, J = 7.9, H Ar); 6.22 (2H, s, CH2); 3.99 (3H, s, OCH3); 3.89 (3H, s, OCH3). 13C NMR spectrum, δ, ppm: 160.3; 158.4; 147.5; 140.5; 134.2; 130.2; 129.6; 128.9; 125.4; 53.6; 52.6; 51.1. Found, m/z: 343.0649 [M+Na]+. C13H12N4NaO6. Calculated, m/z: 343.0649.
Dimethyl 1-[(3-nitrophenyl)methyl]-1H-1,2,3-triazole-4,5-dicarboxylate (2l).36 Yield 275 mg (86%), yellow liquid. IR spectrum, ν, cm–1: 3075, 2958, 1730, 1552, 1521. 1H NMR spectrum, δ, ppm: 8.25–8.22 (2H, m, H Ar); 7.68–7.54 (2H, m, H Ar); 5.94 (2H, s, CH2); 3.99 (3H, s, OCH3); 3.96 (3H, s, OCH3). 13C NMR spectrum, δ, ppm: 160.2; 158.5; 148.4; 140.6; 135.9; 134.2; 130.1; 129.2; 123.8; 123.2; 53.5; 52.8; 52.4. Found, m/z: 343.0649 [M+Na]+. C13H12N4NaO6. Calculated, m/z: 343.0649.
Dimethyl 1-[(4-nitrophenyl)methyl]-1H-1,2,3-triazole-4,5-dicarboxylate (2m).37 Yield 285 mg (89%), white solid, mp 91–92°C. IR spectrum, ν, cm–1: 2952, 1724, 1547, 1472. 1H NMR spectrum, δ, ppm (J, Hz): 8.20 (2H, d, AA'XX' system, J = 4.6, H Ar); 7.43 (2H, d, AA'XX' system, J = 11.5, H Ar); 5.90 (2H, s, CH2); 3.92 (3H, s, OCH3); 3.87 (3H, s, OCH3). 13C NMR spectrum, δ, ppm: 160.2; 158.5; 148.0; 140.7; 129.1; 128.9; 124.1; 53.5; 52.9; 52.8. Found, m/z: 343.0649 [M+Na]+. C13H12N4NaO6. Calculated, m/z: 343.0649.
Synthesis of methyl carboxylates 3a–m and 4a–m (General method). The reaction was performed under argon atmosphere. A solution of ester 2a–m (1.0 equiv) in anhydrous THF (15 ml) was stirred at –10 to –5°C for 30 min followed by dropwise addition of LTBA (1.8 equiv, 1 M solution in THF). Upon completion, the reaction mixture was quenched with ice-cold water. Then, the mixture was filtered through Nutsche filter with silica gel and washed with EtOAc. The crude reaction mixture was purified by column chromatography on silica gel using EtOAc–hexane, 6:4 as eluent.
Methyl 1-benzyl-5-formyl-1H-1,2,3-triazole-4-carboxy-late (3a). Yield 172 mg (70%), white solid, mp 73–74°C. IR spectrum, ν, cm–1: 3005, 2951, 2845, 1714, 1690, 1552, 1463. 1H NMR spectrum, δ, ppm: 10.47 (1H, s, CHO); 7.32 (5H, m, H Ar); 5.92 (2H, s, CH2); 4.03 (3H, s, OCH3). 13C NMR spectrum, δ, ppm: 180.9 (CHO); 160.6 (CO2CH3); 142.5; 133.8; 133.6; 128.8 (2C); 128.3; 54.3; 52.9. Found, m/z: 244.0720 [M–H]–. C12H10N3O3. Calculated, m/z: 244.0728.
Methyl 1-[(2-fluorophenyl)methyl]-5-formyl-1H-1,2,3-triazole-4-carboxylate (3b). Yield 197 mg (75%), white solid, mp 55–56°C. IR spectrum, ν, cm–1: 3018, 2955, 2842, 1726, 1694, 1589, 1493. 1H NMR spectrum, δ, ppm: 10.50 (1H, s, CHO); 7.41–6.90 (4H, m, H Ar); 6.01 (2H, s, CH2); 4.06 (3H, s, OCH3). 13C NMR spectrum, δ, ppm (J, Hz): 180.9 (CHO); 160.6 (CO2CH3); 160.5 (d, J = 249.5); 142.3; 130.7 (d, J = 8.2); 130.0; 129.5 (d, J = 3.0); 124.5 (d, J = 3.6); 121.2 (d, J = 14.3); 115.8 (d, J = 21.1); 52.9; 48.3. Found, m/z: 262.0615 [M–H]–. C12H9FN3O3. Calculated, m/z: 262.0633.
Methyl 1-[(3-fluorophenyl)methyl]-5-formyl-1H-1,2,3-triazole-4-carboxylate (3c). Yield 187 mg (71%), white solid, mp 102–104°C. IR spectrum, ν, cm–1: 3037, 2960, 2848, 1735, 1681, 1586, 1450. 1H NMR spectrum, δ, ppm (J, Hz): 10.48 (1H, s, CHO); 7.37–7.24 (1H, m, H Ar); 7.14 (1H, d, J = 7.7, H Ar); 7.09–6.92 (2H, m, H Ar); 5.91 (2H, s, CH2); 4.04 (3H, s, OCH3). 13C NMR spectrum, δ, ppm (J, Hz): 180.9; 162.7 (d, J = 247.6); 160.6; 142.5; 136.1; 133.4; 130.5 (d, J = 8.2); 123.9 (d, J = 3.1); 115.8 (d, J = 21.0); 115.3 (d, J = 22.5); 53.6; 52.9. Found, m/z: 262.0643 [M–H]–. C12H9FN3O3. Calculated, m/z: 262.0633.
Methyl 1-[(4-fluorophenyl)methyl]-5-formyl-1H-1,2,3-triazole-4-carboxylate (3d). Yield 184 mg (70%), white solid, mp 78–79°C. IR spectrum, ν, cm–1: 3089, 2957, 2853, 1713, 1688, 1558, 1467. 1H NMR spectrum, δ, ppm (J, Hz): 10.48 (1H, s, CHO); 7.39 (2H, dd, J = 8.8, J = 5.2, H Ar); 7.02 (2H, t, J = 8.7, H Ar); 5.88 (2H, s, CH2); 4.03 (3H, s, OCH3). 13C NMR spectrum, δ, ppm (J, Hz): 180.2 (CHO); 162.1 (d, J = 248.6); 160.4 (CO2CH3); 141.8; 132.7; 129.6 (d, J = 8.5); 128.8 (d, J = 3.6); 115.1 (d, J = 21.7); 52.8; 52.1. Found, m/z: 262.0637 [M–H]–. C12H9FN3O3. Calculated, m/z: 262.0633.
Methyl 1-[(2-chlorophenyl)methyl]-5-formyl-1H-1,2,3-triazole-4-carboxylate (3e). Yield 238 mg (85%), white solid, mp 71–72°C. IR spectrum, ν, cm–1: 3001, 2947, 1746, 1691, 1537, 1459. 1H NMR spectrum, δ, ppm (J, Hz):
10.50 (1H, s, CHO); 7.42 (1H, d, J = 10.3, H Ar); 7.33–7.12 (2H, m, H Ar); 6.77 (1H, d, J = 8.7, H Ar); 6.05 (2H, s, CH2); 4.05 (3H, s, OCH3). 13C NMR spectrum, δ, ppm: 180.9 (CHO); 160.6 (CO2CH3); 142.3; 133.2; 131.9; 129.9; 129.8; 128.4; 127.2; 53.0; 52.0. Found, m/z: 302.0305 [M+Na]+. C12H10ClN3NaO3. Calculated, m/z: 302.0303.
Methyl 1-[(3-chlorophenyl)methyl]-5-formyl-1H-1,2,3-triazole-4-carboxylate (3f). Yield 202 mg (72%), white solid, mp 104–106°C. IR spectrum, ν, cm–1: 3020, 2956, 2853, 1709, 1687, 1577, 1463. 1H NMR spectrum, δ, ppm: 10.48 (1H, s, CHO); 7.29–7.25 (4H, m, H Ar); 5.89 (2H, s, CH2); 4.04 (3H, s, OCH3). 13C NMR spectrum, δ, ppm: 180.2; 159.7; 141.8; 134.9; 133.9; 132.89; 129.4; 128.3; 127.5; 125.7; 52.8; 52.1. Found, m/z: 302.0299 [M+Na]+. C12H10ClN3NaO3. Calculated, m/z: 302.0303.
Methyl 1-[(4-chlorophenyl)methyl]-5-formyl-1H-1,2,3-triazole-4-carboxylate (3g). Yield 196 mg (70%), white solid, mp 89–91°C. IR spectrum, ν, cm–1: 3006, 2954, 2851, 1715, 1686, 1555, 1491. 1H NMR spectrum, δ, ppm: 10.47 (1H, s, CHO); 7.31 (4H, s, H Ar); 5.88 (2H, s, CH2); 4.01 (3H, s, OCH3). 13C NMR spectrum, δ, ppm: 181.0; 160.5; 142.6; 134.9; 133.5; 132.4; 129.7; 129.1; 53.8; 52.8. Found, m/z: 278.0338 [M–H]–. C12H9ClN3O3. Calculated, m/z: 278.0338.
Methyl 5-formyl-1-[(2-methoxyphenyl)methyl]-1H-1,2,3-triazole-4-carboxylate (3h). Yield 193 mg (70%), white solid, mp 100–101°C. IR spectrum, ν, cm–1: 3020, 2923, 2831, 1730, 1699, 1591, 1495. 1H NMR spectrum, δ, ppm: 10.53 (1H, s, CHO); 7.43–7.19 (1H, m, H Ar); 6.99–6.65 (2H, m, H Ar); 5.95 (2H, s, CH2); 4.04 (3H, s, OCH3); 3.82 (3H, s, OCH3). 13C NMR spectrum, δ, ppm: 180.1; 160.1; 156.4; 141.4; 133.7; 129.2; 128.1; 121.8; 119.9; 109.9; 54.8; 52.1; 49.5. Found, m/z: 298.0802 [M+Na]+. C13H13N3NaO4. Calculated, m/z: 298.0798.
Methyl 5-formyl-1-[(3-methoxyphenyl)methyl]-1H-1,2,3-triazole-4-carboxylate (3i). Yield 195 mg (71%), white solid, mp 54–55°C. IR spectrum, ν, cm–1: 3015, 2954, 2851, 1721, 1693, 1601, 1490. 1H NMR spectrum, δ, ppm: 10.43 (1H, s, CHO); 7.22–7.17 (2H, m, H Ar); 6.90–6.79 (2H, m, H Ar); 5.85 (2H, s, CH2); 4.00 (3H, s, OCH3); 3.74 (3H, s, OCH3). 13C NMR spectrum, δ, ppm: 180.9; 160.5; 159.8; 142.4; 135.3; 133.7; 129.9; 120.4; 114.2; 113.7; 55.2; 54.1; 52.8. Found, m/z: 298.0790 [M+Na]+. C13H13N3NaO4. Calculated, m/z: 298.0798.
Methyl 5-formyl-1-[(4-methoxyphenyl)methyl]-1H-1,2,3-triazole-4-carboxylate (3j). Yield 190 mg (69%), white solid, mp 88–89°C. IR spectrum, ν, cm–1: 3003, 2954, 2839, 1727, 1694, 1611, 1513. 1H NMR spectrum, δ, ppm (J, Hz): 10.47 (1H, s, CHO); 7.36 (2H, d, AA'XX' system, J = 8.7, H Ar); 6.84 (2H, d, AA'XX' system, J = 8.7, H Ar); 5.85 (2H, s, CH2); 4.03 (3H, s, OCH3); 3.78 (3H, s, OCH3). 13C NMR spectrum (75 MHz, CDCl3), δ, ppm: 181.1; 160.7; 159.9; 142.6; 133.6; 130.0; 126.0; 114.2; 55.3; 53.9; 52.9. Found, m/z: 298.0797 [M+Na]+. C13H13N3NaO4. Calculated, m/z: 298.0798.
Methyl 5-formyl-1-[(2-nitrophenyl)methyl]-1H-1,2,3-triazole-4-carboxylate (3k). Yield 258 mg (89%), white solid, mp 111–112°C. IR spectrum, ν, cm–1: 2955, 2923, 2853, 1730, 1694, 1524, 1460. 1H NMR spectrum, δ, ppm: 10.46 (1H, s, CHO); 8.16 (1H, m, H Ar); 7.56–7.52 (2H, m, H Ar); 6.78–6.74 (1H, m, H Ar); 6.22 (2H, s, CH2); 4.07 (3H, s, OCH3). 13C NMR spectrum, δ, ppm: 179.9; 159.5; 146.8; 141.5; 133.2; 128.9; 128.6; 127.4; 124.6; 52.2; 50.9. Found, m/z: 313.0548 [M+Na]+. C12H10N4NaO5. Calculated, m/z: 313.0543.
Methyl 5-formyl-1-[(3-nitrophenyl)methyl]-1H-1,2,3-triazole-4-carboxylate (3l). Yield 211 mg (73%), white solid, mp 99–100°C. IR spectrum, ν, cm–1: 3119, 3029, 2959, 1730, 1691, 1532, 1461. 1H NMR spectrum, δ, ppm (J, Hz): 10.49 (1H, s, CHO); 8.25 (1H, s, H Ar); 8.21 (1H, d, J = 8.1, H Ar); 7.72 (1H, d, J = 7.7, H Ar); 7.57 (1H, dd, J = 9.9, J = 6.0, H Ar); 6.03 (2H, s, CH2); 4.06 (3H, s, OCH3). 13C NMR spectrum, δ, ppm: 181.1; 160.4; 148.5; 142.7; 135.7; 134.4; 133.7; 130.1; 123.9; 123.4; 53.4; 53.1. Found, m/z: 313.0547 [M+Na]+. C12H10N4NaO5. Calculated, m/z: 313.0543.
Methyl 5-formyl-1-(4-nitrophenyl)methyl]-1H-1,2,3-triazole-4-carboxylate (3m). Yield 218 mg (75%), white solid, mp 95–97°C. IR spectrum, ν, cm–1: 3068, 2989, 1722, 1687, 1515, 1494, 1470. 1H NMR spectrum, δ, ppm (J, Hz): 10.48 (1H, s, CHO); 8.21 (2H, d, J = 8.6, H Ar); 7.53 (2H, d, J = 8.5, H Ar); 6.01 (2H, s, CH2); 4.05 (3H, s, OCH3). 13C NMR spectrum (75 MHz, CDCl3), δ, ppm: 180.2; 159.6; 147.4; 141.9; 139.7; 132.9; 128.4; 123.3; 52.6; 52.3. Found, m/z: 313.0543 [M+Na]+. C12H10N4NaO5. Calculated, m/z: 313.0543.
Methyl 1-benzyl-5-(hydroxymethyl)-1H-1,2,3-triazole-4-carboxylate (4a).39 Yield 64 mg (26%), white solid, mp 84–85°C. IR spectrum, ν, cm–1: 3230, 3030, 2957, 1710, 1570, 1495. 1H NMR spectrum, δ, ppm (J, Hz): 7.36–7.20 (5H, m, H Ar); 5.66 (2H, s, CH2); 4.78 (2H, d, J = 6.9, CH2OH); 4.00 (3H, s, OCH3); 3.71 (1H, t, J = 7.0, OH). 13C NMR spectrum, δ, ppm: 163.0; 141.2; 137.4; 133.9; 129.0; 128.8; 127.3; 53.3; 52.7; 52.6. Found, m/z: 270.0849 [M+Na]+. C12H13N3NaO3. Calculated, m/z: 270.0849.
Methyl 1-[(2-fluorophenyl)methyl]-5-(hydroxymethyl)-1H-1,2,3-triazole-4-carboxylate (4b). Yield 52 mg (20%), white solid, mp 93–94°C. IR spectrum, ν, cm–1: 3247, 2956, 1710, 1591, 1495. 1H NMR spectrum, δ, ppm: 7.32–7.09 (4H, m, H Ar); 5.70 (2H, s, CH2); 4.88 (2H, s, CH2OH); 4.01 (3H, s, OCH3); 3.80 (1H, s, OH). 13C NMR spectrum, δ, ppm (J, Hz): 163.1; 159.9 (d, J = 247.5); 141.4; 137.2; 130.9 (d, J = 8.2); 129.7 (d, J = 3.0); 124.9 (d, J = 3.8); 121.2 (d, J = 14.3); 115.9 (d, J = 21.1); 53.3; 52.7; 45.9. Found, m/z: 288.0755 [M+Na]+. C12H12FN3NaO3. Calculated, m/z: 288.0755.
Methyl 1-[(3-fluorophenyl)methyl]-5-(hydroxymethyl)-1H-1,2,3-triazole-4-carboxylate (4c). Yield 58 mg (22%), white solid, mp 115–117°C. IR spectrum, ν, cm–1: 3243, 2950, 1716, 1612, 1584. 1H NMR spectrum, δ, ppm (J, Hz): 7.33 (1H, dd, J = 13.8, J = 8.0, H Ar); 7.02 (2H, dd, J = 13.5, J = 7.9, H Ar); 6.92 (1H, d, J = 9.2, H Ar); 5.66 (2H, s, CH2); 4.82 (2H, s, CH2OH); 3.99 (3H, s, OCH3); 3.68 (1H, s, OH). 13C NMR spectrum, δ, ppm (J, Hz): 162.9 (d, J = 248.1); 162.5; 141.3; 137.1; 136.3 (d, J = 17.3); 130.8 (d, J = 8.3); 123.0 (d, J = 3.0); 115.8 (d, J = 21.0); 114.6 (d, J = 22.5); 52.9; 52.5; 52.2. Found, m/z: 288.0743 [M+Na]+. C12H12FN3NaO3. Calculated, m/z: 288.0755.
Methyl 1-[(4-fluorophenyl)methyl]-5-(hydroxymethyl)-1H-1,2,3-triazole-4-carboxylate (4d). Yield 29 mg (11%), white solid, mp 97–98°C. IR spectrum, ν, cm–1: 3239, 2956, 1711, 1633, 1570. 1H NMR spectrum, δ, ppm (J, Hz): 7.21–7.08 (2H, m, H Ar); 7.05 (2H, t, J = 8.6, H Ar); 5.62 (2H, s, CH2); 4.81 (2H, d, J = 6.9, CH2OH); 3.63 (1H, t, J = 7.0, OH); 3.99 (3H, s, OCH3). 13C NMR spectrum, δ, ppm (J, Hz): 158.2; 158.1 (d, J = 248.4); 124.6 (d, J = 8.5); 111.5 (d, J = 21.7); 136.2; 132.6; 125.1; 48.6; 47.9; 47.2. Found, m/z: 288.0755 [M+Na]+. C12H12FN3NaO3. Calculated, m/z: 288.0755.
Methyl 1-[(2-chlorophenyl)methyl]-5-(hydroxymethyl)-1H-1,2,3-triazole-4-carboxylate (4e). Yield 33 mg (12%), white solid, mp 88–89°C. IR spectrum, ν, cm–1: 3233, 2956, 1711, 1570, 1470. 1H NMR spectrum, δ, ppm (J, Hz): 7.44–6.85 (4H, m, H Ar); 5.77 (2H, s, CH2); 4.83 (2H, d, J = 6.5, CH2OH); 3.86 (3H, s, OCH3); 3.85 (1H, t, J = 7.2, OH). 13C NMR spectrum, δ, ppm: 162.9; 141.6; 137.1; 132.6; 131.9; 129.9; 129.8; 128.6; 127.6; 53.2; 52.6; 49.8. Found, m/z: 304.0460 [M+Na]+. C12H12ClN3NaO3. Calculated, m/z: 304.0459.
Methyl 1-[(3-chlorophenyl)methyl]-5-(hydroxymethyl)-1H-1,2,3-triazole-4-carboxylate (4f). Yield 39 mg (14%), white solid, mp 109–111°C. IR spectrum, ν, cm–1: 3289, 2917, 1720, 1578, 1433. 1H NMR spectrum, δ, ppm (J, Hz): 7.31–7.08 (4H, m, H Ar); 5.64 (2H, s, CH2); 4.82 (1H, d, J = 6.8, CH2OH); 3.99 (3H, s, OCH3); 3.65 (1H, t, J = 6.8, OH). 13C NMR spectrum, δ, ppm: 162.4; 140.8; 136.9; 135.9; 134.9; 130.3; 128.9; 127.5; 125.5; 53.0; 52.4; 51.9. Found, m/z: 304.04590 [M+Na]+. C12H12ClN3NaO3. Calculated, m/z: 304.0459.
Methyl 1-[(4-chlorophenyl)methyl]-5-(hydroxymethyl)-1H-1,2,3-triazole-4-carboxylate (4g). Yield 59 mg (21%), white solid, mp 127–129°C. IR spectrum, ν, cm–1: 3214, 2957, 1711, 1570, 1491. 1H NMR spectrum, δ, ppm (J, Hz): 7.33 (2H, d, AA'XX' system, J = 8.5, H Ar); 7.17 (2H, d, AA'XX' system, J = 8.4, H Ar); 5.62 (2H, s, CH2); 4.81 (2H, d, J = 4.5, CH2OH); 3.99 (3H, s, OCH3); 3.62 (1H, s, OH). 13C NMR spectrum, δ, ppm: 162.7; 140.8; 137.0; 134.8; 132.5; 129.3; 129.1; 53.1; 52.5; 51.9. Found, m/z: 304.0458 [M+Na]+. C12H12ClN3NaO3. Calculated, m/z: 304.0459.
Methyl 5-(hydroxymethyl)-1-[(2-methoxyphenyl)methyl]-1H-1,2,3-triazole-4-carboxylate (4h). Yield 77 mg (28%), white solid, mp 115–117°C. IR spectrum, ν, cm–1: 3356, 2952, 1723, 1603, 1496. 1H NMR spectrum, δ, ppm (J, Hz): 7.28 (1H, t, J = 7.8, H Ar); 7.03 (1H, d, J = 7.5, H Ar); 6.88 (2H, t, J = 8.4, H Ar); 5.60 (2H, s, CH2); 4.85 (2H, d, J = 5.5, CH2OH); 4.20 (1H, t, J = 6.0, OH); 3.94 (3H, s, OCH3); 3.83 (3H, s, OCH3). 13C NMR spectrum, δ, ppm: 163.2; 156.9; 141.5; 137.2; 130.5; 129.5; 122.2; 120.8; 110.5; 55.5; 53.5; 52.5; 47.8. Found, m/z: 300.0957 [M+Na]+. C13H15N3NaO4. Calculated, m/z: 300.0955.
Methyl 5-(hydroxymethyl)-1-[(3-methoxyphenyl)methyl]-1H-1,2,3-triazole-4-carboxylate (4i). Yield 47 mg (17%), white solid, mp 110–111°C. IR spectrum, ν, cm–1: 3274, 2955, 1714, 1531, 1459. 1H NMR spectrum, δ, ppm: 7.28–7.23 (1H, m, H Ar); 6.86–6.72 (3H, m, H Ar); 5.62 (2H, s, CH2); 4.80 (2H, s CH2OH); 3.97 (3H, s, OCH3); 3.76 (3H, s, OCH3). 13C NMR spectrum, δ, ppm: 162.5; 160.0; 140.9; 136.8; 135.5; 130.1; 119.6; 114.0; 113.1; 55.2; 52.9; 52.6; 52.4. Found, m/z: 300.0960 [M+Na]+. C13H15N3NaO4. Calculated, m/z: 300.0955.
Methyl 5-(hydroxymethyl)-1-[(4-methoxyphenyl)methyl]-1H-1,2,3-triazole-4-carboxylate (4j).37 Yield 63 mg (23%), white solid, mp 104–105°C. IR spectrum, ν, cm–1: 3241, 2950, 1715, 1610, 1568. 1H NMR spectrum, δ, ppm (J, Hz): 7.18 (2H, d, AA'XX' system, J = 8.6, H Ar); 6.87 (2H, d, AA'XX' system, J = 8.7, H Ar); 5.59 (2H, s, CH2); 4.80 (2H, d, J = 6.6, CH2OH); 3.99 (3H, s, OCH3); 3.80 (3H, s, OCH3); 3.76 (1H, t, J = 6.7, OH). 13C NMR spectrum, δ, ppm: 163.2; 159.9; 141.2; 129.5; 125.9 (2C); 114.8; 55.5; 53.5; 52.8; 52.5. Found, m/z: 300.0959 [M+Na]+. C13H15N3NaO4. Calculated, m/z: 300.0955.
Methyl 5-(hydroxymethyl)-1-[(2-nitrophenyl)methyl]-1H-1,2,3-triazole-4-carboxylate (4k). Yield 14 mg (5%), white solid, mp 152–153°C. IR spectrum, ν, cm–1: 3241, 2952, 1712, 1611, 1528. 1H NMR spectrum, δ, ppm (J, Hz): 8.20 (1H, d, J = 9.6, H Ar); 7.66–7.49 (2H, m, H Ar); 6.77 (1H, d, J = 9.4, H Ar); 6.09 (2H, s, CH2); 4.90 (2H, s, CH2OH); 4.01 (3H, s, OCH3); 3.55 (1H, s, OH). 13C NMR spectrum, δ, ppm: 162.7; 147.1; 141.7; 137.2; 134.4; 130.3; 129.5; 128.8; 125.6; 53.3; 52.7; 49.4. Found, m/z: 315.0703 [M+Na]+. C12H12N4NaO5. Calculated, m/z: 315.0700.
Methyl 5-(hydroxymethyl)-1-[(4-nitrophenyl)methyl]-1H-1,2,3-triazole-4-carboxylate (4l). Yield 27 mg (9%), white solid, mp 116–117°C. IR spectrum, ν, cm–1: 3269, 2956, 1716, 1531, 1460. 1H NMR spectrum, δ, ppm: 8.22–8.16 (2H, m, H Ar); 7.59–7.56 (2H, m, H Ar); 5.78 (2H, s, CH2); 4.91 (2H, s, CH2OH); 3.98 (3H, s, OCH3); 3.54 (1H, s, OH). 13C NMR spectrum, δ, ppm: 161.6; 147.7; 140.0; 136.3; 135.3; 132.8; 129.8; 122.9; 121.9; 52.4; 51.8; 50.9. Found, m/z: 315.0707 [M+Na]+. C12H12N4NaO5. Calculated, m/z: 315.0700.
Methyl 5-(hydroxymethyl)-1-(3-nitrophenyl)methyl]-1H-1,2,3-triazole-4-carboxylate (4m). Yield 55 mg (19%), white solid, mp 164–166°C. IR spectrum, ν, cm–1: 3404, 2956, 1707, 1563, 1512, 1468. 1H NMR spectrum, δ, ppm (J, Hz): 8.24 (1H, d, J = 8.9, H Ar); 7.42 (1H, d, J = 8.9, H Ar); 5.77 (2H, s, CH2); 4.88 (2H, d, J = 7.7, CH2OH); 4.02 (3H, s, OCH3); 3.38 (1H, t, J = 6.8, OH). 13C NMR spectrum, δ, ppm: 161.6; 147.3; 140.1 (2C); 136.4; 127.5; 123.5; 52.4; 51.8; 50.9. Found, m/z: 315.0699 [M+Na]+. C12H12N4NaO5. Calculated, m/z: 315.0700.
X-ray structural study of compounds 3g,l were obtained on a Bruker APEX II QUAZAR three-circle diffractometer using monochromatized MoKα X-radiation (λ 0.71073 Å) after crystallization from CH2Cl2–hexane solvent mixture. Indexing, data collection, data reduction (SAINT, version 8.34A)40 and absorption correction (SADABS, version2014/5)41 were carried out using APEX2.42 All crystal structures were solved using SHELXT and then refined by full-matrix least-squares refinements on F2 using the SHELXL in Olex2 software package.43,44 The aromatic and aliphatic C-bound H atoms were positioned geometrically and refined using a riding mode. Crystal structure validations and geometrical calculations were performed using Platon.45 Additional crystallographic data have been deposited within the Cambridge Crystallographic Data Center (deposits CCDC 2172914 (compound 3g) and CCDC 2172913 (compound 3l)).
Supplementary information file, containing FTIR, 1H, 13C NMR and HRMS spectra of the synthesized compounds, and X-ray crystallography data for compounds 3g,l is available at the journal website http://springerlink.bibliotecabuap.elogim.com/ journal/10593.
We are grateful to the Scientific and Technological Research Council of Turkey (120Z876) for financial support. We thank Dr. Ahmet Ceyhan Gören (GTU) for 2D NMR data and Dr. Çağatay Dengiz (METU) for reviewing the manuscript.
References
Buckle, D. R.; Rockell, C. J. M; Smith, H.; Spicer, B. A. J. Med. Chem. 1986, 29, 2262.
Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Chem. Rev. 2013, 113, 4905.
Lauria, A.; Delisi, R.; Mingoia, F.; Terenzi, A.; Martorana, A.; Barone, G.; Almerico, A. M. Eur. J. Org. Chem. 2014, 3289.
Nguyen, D. T.; Ngo, T. H.; Tran, H. T.; Dinh, T. P.; Do, P. T.; Nguyen, H. B.; Tran, L. T. P.; Ta, H. M. Chem. Heterocycl. Compd. 2021, 57, 1037.
Bozorov, K.; Zhao, J.; Aisa, H. A. Bioorg. Med. Chem. 2019, 27, 3511.
Tapkir, S. R.; Patil, R. H.; Galave, S. A.; Phadtare, G. R.; Khedkar, V. M.; Garud, D. R. J. Heterocycl. Chem. 2022, 59, 739.
Oubella, A.; Bimoussa, A.; Byadi, S.; Fawzi, M.; Laamari, Y.; Auhmani, A.; Morjani, H.; Robert, A.; Riahi, A.; Itto, M. Y. A. J. Mol. Struct. 2022, 1265, 133383.
Huisgen, R. Angew. Chem., Int. Ed. 1963, 2, 565.
Dheer, D.; Singh, V.; Shankar, R. Bioorg. Chem. 2017, 71, 30.
Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004.
Boren, B. C.; Narayan, S.; Rasmussen, L. K.; Zhang, L.; Zhao, H.; Lin, Z.; Jia, G.; Fokin, V. V. J. Am. Chem. Soc. 2008, 130, 8923.
Li, L.; Shang, T.; Ma, X.; Guo, H.; Zhu, A.; Zhang, G. Synlett 2015, 26, 695.
Chuprakov, S.; Chernyak, N.; Dudnik A. S.; Gevorgyan, V. Org. Lett. 2007, 9, 2333.
Shiri, P.; Amani, A. M.; Mayer-Gall, T. Beilstein J. Org. Chem. 2021, 17, 1600.
Ramanaiah, K. C. V.; Stevens, E. D.; Trudell, M. L.; Pagoria, P. F. J. Heterocycl. Chem. 2000, 37, 1597.
Pokhodylo, N. T.; Shyyka, O. Ya.; Obushak, M. D. Chem. Heterocycl. Compd. 2018, 54, 773.
Fariña, F.; Fernández, P.; Fraile, M. T.; Martín, M. V.; Martín, M. R. Heterocycles 1989, 29, 967.
Baykal, A.; Zhang, D.; Knelles, J.; Alt, I. T.; Plietker, B. Asian J. Chem. 2019, 14, 3003.
Jomova, K.; Valko, M. Toxicology 2011, 283, 65.
Gierlich, J.; Burley, G. A.; Gramlich, P. M. E.; Hammond, D. M.; Carell, T. Org. Lett. 2006, 8, 3639.
Kennedy, D. C.; McKay, C. S.; Legault, M. C. B.; Danielson, D. C.; Blake, J. A.; Pegoraro, A. F.; Stolow, A.; Mester, Z.; Pezacki, J. P. J. Am. Chem. Soc. 2011, 133, 17993.
Zheng, H.; McDonald, R.; Hall, D. G. Chem.–Eur. J. 2010, 16, 5454.
Carey, F. A.; Sundberg, R. J. Advanced Organic Chemistry: Part B: Reaction and Synthesis; Springer, 2007.
Pérez-Serrano, L.; Casarrubios, L.; Dominguez, G.; González-Pérez, P.; Pérez-Castells, J. Synthesis 2002, 1810.
Dransfield, P. J.; Dilley, A. S.; Wang, S.; Romo, D. Tetrahedron 2006, 62, 5223.
Devine, W. G.; Diaz-Gonzalez, R.; Ceballos-Perez, G.; Rojas, D.; Satoh, T.; Tear, W.; Ranade, R. M.; Barros-Álvarez, X.; Hol, W. G. J.; Buckner, F. S.; Navarro, M.; Pollastri, M. P. ACS Infect. Dis. 2017, 3, 225.
Lopchuk, J. M.; Gribble, G. W. Heterocycles 2011, 82, 1617.
Deng, Y.; Liang, X.; Wei, K.; Yang, Y. R. J. Am. Chem. Soc. 2021, 143, 20622.
Yoshida, K.; Hayashi, K.; Yanagisawa, A. Org. Lett. 2011, 13, 4762.
Yasui, E.; Tsuda, J.; Ohnuki, S.; Nugumo, S. Chem. Pharm. Bull. 2016, 64, 1262.
Shanmugavelan, P.; Nagarajan, S.; Sathishkumar, M.; Ponnuswamy, A.; Yogeeswari, P.; Sriram, D. Bioorg. Med. Chem. Lett. 2011, 21, 7273.
Shaikh, M. H.; Subhedar, D. D.; Arkile, M.; Khedkar, V. M.; Jadhav, N.; Sarkar, D.; Shingate, B. B. Bioorg. Med. Chem. Lett. 2016, 26, 561.
Sri Ramya, P. V.; Angapelly, S.; Guntuku, L.; Digwal, C. S.; Babu, B. N.; Naidu, V. G. M.; Kamal, A. Eur. J. Med. Chem. 2017, 127, 100.
Rodríguez-Hernández, D.; Demuner, A. J.; Barbosa, L. C. A.; Heller, L.; Csuk, R. Eur. J. Med. Chem. 2016, 115, 257.
Biagi, G.; Giorgi, I.; Livi, O.; Manera, C.; Scartoni, V.; Betti, L.; Giannaccini, G.; Lucacchini, A. Farmaco 1999, 54, 615.
Abu-Orabi, S. T.; Atfah, A. M.; Jibril, I.; Mari'i, F. M.; Ali, A. A. J. Heterocycl. Chem. 1989, 26, 1461.
Barman, M. K.; Sinha, A. K.; Nembenna, S. Green Chem. 2016, 18, 2534.
Tarawneh, A. H.; Al-Momani, L. A.; León, F.; Jain, S. K.; Gadetskaya, V. A.; Abu-Orabi, S. T.; Tekwani, B. L.; Cutler, S. J. Med. Chem. Res. 2018, 27, 1269.
Butler, C. R.; Bendesky, J.; Schoffstall, A. M. Molecules 2012, 26, 5589.
SAINT, version 8.34A; Bruker AXS, Inc.: Madison, 2013.
SADABS, version 2014/5; Bruker AXS, Inc.: Madison, 2014.
APEX2, version 2014.11–0; Bruker AXS, Inc.: Madison, 2014.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
Spek, A. L. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2009, 65, 148.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2023, 59(4/5), 267–276
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Erdemir, G.Y., Altundas, A. Highly regioselective one-step synthesis of 1-benzyl-5-formyl-1,2,3-triazole-4-carboxylates. Chem Heterocycl Comp 59, 267–276 (2023). https://doi.org/10.1007/s10593-023-03192-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-023-03192-0