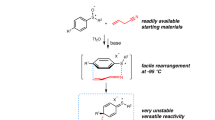
All reported approaches toward synthesis of 1,2-azaphosphetidine 2-oxide/sulfide derivatives are summarized. Synthetic methods for 1,2-azaphosphetidine 2-oxides/sulfides are gathered in four categories: ring expansion of aziridines, cyclizations, carbene insertions, and cycloadditions.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction 
1,2-Azaphosphetidine 2-oxides (β-phospholactams) and 2-sulfides (β-thiophospholactams) are important phosphorus analogs of β-lactams (azetidin-2-ones) and β-sultams (1,2-thiazetidine 1,1-dioxides). They can be considered as transition state analogs of β-lactamase inhibitors.1 Unlike widely investigated β-lactams and β-sultams,2,3 less attention has been paid to β-phospholactams and β-thiophospholactams, though they show some biological importance.1

Ring expansion of aziridines

1-Substituted aziridines react with PCl3 or (EtO)2PCl to give aziridine salts, which decompose to RN(PCl2)CH2CH2Cl or RN[P(OEt)2]CH2CH2Cl, respectively, and then on heating generate substituted 1,2-azaphosphetidine 2-oxides through intramolecular Arbuzov reaction.4 The reaction of N-phosphorylated 1,2-azaphosphetidine 2-oxides with protoncontaining nucleophiles (H2O, EtOH, or PhOH) leads to the ring cleavage across the P–N bond giving rise to 2-aminoethylphosphonic acid derivatives.5


Cyclizations

Treatment of Br(CH2)2NH2·HBr with (RO)2PCl and Et3N under mild conditions gave N-phosphorylated 1,2-azaphosphetidine 2-oxides via the formation of [(RO)2P]2NCH2CH2Br and subsequent Arbuzov reaction. Reaction of N-phosphorylated 1,2-azaphosphetidine 2-oxide (R = Et) with tosyl azide afforded its imino derivative.6


 Jiaxi Xu was born in 1963 in Jilin, China. He received his PhD in 1992 from Department of Chemistry at Peking University in China. After a post-doctoral stay at the School of Pharmaceutical Sciences at Beijing Medical University (Health Center of Peking University now), he was appointed as an associate professor at College of Chemistry and Molecular Engineering at Peking University. He also worked as a visiting scholar in the Department of Chemistry at the Chinese University of Hong Kong (1995–1996), Department of Chemistry at Colorado State University (2000–2001), and Medical School at Vanderbilt University (2001–2002). He was promoted a full professor in 2004. At the end of 2007 he started working at the Faculty of Science (College of Chemistry now) at Beijing University of Chemical Technology. His research interests include synthetic methodologies and related mechanisms, asymmetric synthesis and catalysis, synthesis of heterocyclic compounds, unnaturally occurring amino acids and peptides.
Jiaxi Xu was born in 1963 in Jilin, China. He received his PhD in 1992 from Department of Chemistry at Peking University in China. After a post-doctoral stay at the School of Pharmaceutical Sciences at Beijing Medical University (Health Center of Peking University now), he was appointed as an associate professor at College of Chemistry and Molecular Engineering at Peking University. He also worked as a visiting scholar in the Department of Chemistry at the Chinese University of Hong Kong (1995–1996), Department of Chemistry at Colorado State University (2000–2001), and Medical School at Vanderbilt University (2001–2002). He was promoted a full professor in 2004. At the end of 2007 he started working at the Faculty of Science (College of Chemistry now) at Beijing University of Chemical Technology. His research interests include synthetic methodologies and related mechanisms, asymmetric synthesis and catalysis, synthesis of heterocyclic compounds, unnaturally occurring amino acids and peptides.
Cyclizations (continued) 
Reaction of diethyl phosphorisocyanatidite with mucochloric acid led to a bicyclic 1,2-azaphosphetidine 1-oxide derivative.7 The highly reactive isocyanate fragment at the P atom permits one to effect cyclization after the 1st stage of the Arbuzov reaction, thus extending the synthetic potential of mucochloric acid derivative phosphorylation.

In an effort to test whether a transition state analog is an inhibitor of the metallo-β-lactamases, a phospholactam analog of carbapenem was synthesized from hexanedioic acid.1 It was first converted into β-aminoalkylphosphonic monomethyl ester, which was transformed to the designed fused bicyclic 1,2-azaphosphetidine 2-oxide derivative, phosphacarbapenem, by treatment with SOCl2 in CHCl3 and, then, NaH in the presence of 15-crown-5 in MeCN. The phospholactam proved to be a weak, time-dependent inhibitor at a 100 μM concentration. Docking study was carried out to explain binding and to offer suggestions for modifications of the phospholactam scaffold to improve binding affinities.


Carbene insertions

[Bis(diisopropylamino)phosphanyl](trimethylsilyl)diazomethane (i-Pr2N)2PC(=N2)SiMe3 reacted with p-toluenesulfinyl chloride or p-toluenesulfonyl chloride, affording the same product – 1,2-azaphosphetidine 2-oxide in 85 or 80% yield, respectively.8 These results were rationalized by the transient formation of the carbene (i-Pr2N)2PC(:)S(=O)p-Tol, which rearranged into carbene (i-Pr2N)2P(=O)C(:)Sp-Tol via an intramolecular Wittig-type reaction involving the multiple bond character of phosphanylcarbenes. [Bis(diisopropylamino) thiophosphoranyl](trimethylsilyl)diazomethane reacted with p-TolSCl giving 1,2-azaphosphetidine 2-sulfide in 86% yield. The same derivative was also obtained by photolysis of the C-thiophosphoranyl C-sulfanyl phosphorus ylide Ph3P=C(Sp-Tol)P(=S)(Ni-Pr2)2.
Refluxing of bis[bis(diisopropylamino)phosphinyl]diazomethane in PhH for 48 h quantitatively led to the corresponding azaphosphetane as the only one diastereoisomer. This result was explained in terms of carbene insertion into the methine C–H bond of an isopropyl substituent. Treatment with S8 afforded the corresponding 1,2-azaphosphetidine 2-sulfide.9
UV irradiation of thiophosphoranyl-substituted (triphenylphosphoranylidene) methanes yielded Ph3P and 1,2-azaphosphetidine 2-sulfides.10 The formation of these heterocycles was explained by invoking insertion of the intermediate carbenes into the methine C–H bond of one of the isopropyl groups.
Photolytic or Rh-catalyzed decomposition of α-diazo- β-ketophosphonamidates and subsequent intramolecular C–H insertion of the resulting carbene intermediates gave mono and bicyclic 1,2-azaphosphetidine 2-oxides.11


Cycloadditions

Vacuum thermolysis of Me3SiP=C(OSiMe3)C6H2Me3-2,4,6 provided 3,5-dimesityl-1,2,4-oxadiphosphole, which reacted with tri-tert-butylazete to furnish fused polycyclic 1,2-azaphosphetidine 2-oxide derivative in 41% yield.12 The reaction started with hetero-Diels–Alder cycloaddition followed by rearrangement, phospha-ane reaction, 4-electron electrocyclic ring opening, 6-electron electrocyclic ring opening, [1,5]-H sigmatropic shift, and [2+2] cycloaddition sequence.
1-Alkyl-1,2-diphospholes reacted with N-R-α-phenylnitrones to give, depending on the temperature, either dimers of 1-alkyl-1-oxo-1,2-diphospholes or 1-alkyl-1,7-dioxo- 6-azo-1,7-diphosphabicyclo[3.2.0]hept-2-enes, phosphorus analogs of β-lactams.13 Mixing of 1-alkyl-1,2-diphospholes with nitrones at room temperature led to oxidation products, i.e., 1-alkyl-1-oxo-1,2-diphospholes, and, then, the corresponding dimers. At elevated temperatures, the latter underwent retro-Diels–Alder reaction and subsequent [2+2] cycloaddition with imines generated in the first step, resulting in the formation of fused bicyclic 1,2-azaphosphetidine2-oxides.


References
Yang, K.-W.; Feng, L. J.; Yang, S.-K.; Aitha, M.; LaCuran, A. E.; Oelschlaeger, P.; Crowder, M. W. Bioorg. Med. Chem. Lett. 2013, 23, 5855.
(a) Alcaide, B.; Almendros, P.; Aragoncillo, C. Chem. Rev.2007, 107, 4437. (b) Brandi, A.; Cicchi, S.; Cordero, F. M. Chem. Rev. 2008, 108, 3988. (c) Xu, J. Tetrahedron2012, 68, 10696. (d) Jiao, L.; Liang, Y.; Xu, J. J. Am. Chem. Soc. 2006, 128, 6060. (e) Liang, Y.; Jiao, L.; Zhang, S. W.; Yu, Z.-X.; Xu, J. X. J. Am. Chem. Soc. 2009, 131, 1542. (f) Qi, H.; Li, X.; Xu, J. Org. Biomol. Chem. 2011, 9, 2702.
(a) Chanet-Ray, J.; Vessiere, R. Org. Prep. Proced. Int.1986, 18, 157. (b) Yang, Z.; Xu, J. J. Org. Chem. 2014, 79, 10703. (c) Yang, Z.; Chen, N.; Xu, J. J. Org. Chem. 2015, 80, 3611. (d) Wu, Q.; Yang, Z.; Xu, J. Org. Biomol. Chem. 2016, 14, 7258. (e) Yang, Z.; Xu, J. Tetrahedron2015, 71, 2844. (f) Yang, Z.; Xu, J. RSC Adv.2015, 5, 78396. (g) Xu, W.; Fu, Z.; Yang, Z.; Zhang, L.; Xu, J. Phosphorus, Sulfur Silicon Relat. Elem.2018, 193, 335.
Gubnitskaya, E. S.; Semashko, Z. T.; Parkhomenko, V. S.; Kirsanov, A. V. Zh. Obshch. Khim.1980, 50, 2171.
Gubnitskaya, E. S.; Peresypkina, L. P.; Parkhomenko, V. S. Zh. Obshch. Khim.1986, 56, 2017.
Gubnitskaya, E. S.; Parkhomenko, V. S.; Semashko, Z. T.; Samaray, L. I. Phosphorus, Sulfur Silicon Relat. Elem. 1983, 15, 257.
Polezhaeva, N. A.; Volodina, Yu. M.; Sakhibullina, V. G.; Loginova, I. V.; Galkin, V. I.; Cherkasov, R. A. Russ. J. Gen. Chem.2000, 70, 851.
Sicard, G.; Gruetzmacher, H.; Baceiredo, A.; Fischer, J.; Bertrand, G. J. Org. Chem. 1989, 54, 4426.
Menu, M.-J.; Dartiguenave, Y.; Dartiguenave, M.; Baceiredo, A.; Bertrand, G. Phosphorus, Sulfur Silicon Relat. Elem.1990, 47, 327.
Gruetzmacher, H. Z. Naturforsch. B1990, 45, 170.
Afarinkia, K.; Cadogan, J. I. G.; Rees, C. W. J. Chem. Soc., Chem. Commun. 1992, 3, 285.
Mack, A.; Bergstraesser, U.; Reiss, G. J.; Regitz, M. Eur. J. Org. Chem. 1999, 3, 587.
Zagidullin, A.; Ganushevich, Y.; Miluykov, V.; Krivolapov, D.; Kataeva, O.; Sinyashin, O.; Hey-Hawkins, E. Org. Biomol. Chem. 2012, 10, 5298.
Financial support by the National Natural Science Foundation of China (Nos. 21572017 and 21772010) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(3), 308–310
Rights and permissions
About this article
Cite this article
Xu, J.  Synthesis of 1,2-azaphosphetidine 2-oxides/sulfides (microreview).
Chem Heterocycl Comp 56, 308–310 (2020). https://doi.org/10.1007/s10593-020-02660-1
Synthesis of 1,2-azaphosphetidine 2-oxides/sulfides (microreview).
Chem Heterocycl Comp 56, 308–310 (2020). https://doi.org/10.1007/s10593-020-02660-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02660-1