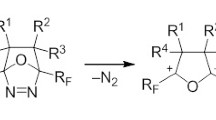
Five- and six-membered fluorine-containing azaheterocycles were synthesized based on available furan-3(2H)-ones, and the influence of the nature of the fluoroalkyl substituent on the direction of the chemical transformations by the action of N,N- and N,O-binucleophiles was revealed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The increased interest in fluorine chemistry in the search for new drugs and promising materials is due to the significant influence of fluorine-containing substituents on the physicochemical properties of the molecule.1 Additionally, the introduction of fluorine atom(s) into the structures of organic compounds leads to an increase in their reactivity which makes new transformations possible that are different from those characteristic of hydrocarbon analogs.2 The widely used fluorine-containing building blocks include unsaturated compounds, di- or tricarbonyl compounds, as well as their analogs.3 Trifluoromethylfunctionalized compounds are the most investigated, the reactions of them with mono- and binucleophiles lead to various acyclic and heterocyclic derivatives.2,3 However, in recent decades, special attention was paid to the strategy of introduction in the various structures of organic compounds of fluorine-containing groups and studying of their influence on the reactivity and properties of the molecule.4
We have previously developed a method for the synthesis of functionalized lithium diketonates 1a,b and furan-3(2H)-ones 2a,b, which have promising coordinating capabilities and reactivity, since they are hidden analogs of 1,2,4-triketones (Scheme 1).5 However, further transformations into substituted pyrazoles, isoxazolines, quinoxalines, and furo[2,3-d]imidazol-2-ones were studied exclusively for trifluoromethyl-containing derivatives 1a and 2a.5 In this work, we replaced the CF3 substituent (compound 2a) with C2F5 (compound 2b) and investigated similarity and differences in the chemical transformations of furan-3(2H)-ones 2a,b by the action of N,N- and N,O-binucleophiles.
It has been established that C2F5-containing analogs 1b and 2b exhibit similar properties with trifluoromethyl analogs5b when conducting reactions with hydrazine, hydroxylamine, and urea in the presence of an acid. The reaction of diketonate 1b and furan-3(2H)-one 2b with these N,N- and N,O-binucleophiles results in functionalized bispyrazole 3, hexahydro-2H-furo[2,3-d]imidazol-2-one 4, and isoxazoline 5 with good yields (Scheme 2).
Benzimidazole 10 was formed as a result of the reaction of o-phenylenediamine with furanone 2b. The same reaction with CF3-analogs led to the formation of substituted quinoxalines.5b,6 Thus, the regioselectivity of reactions in the case of substrate 2b is determined by the initial nucleophilic 1,4-addition at the activated bond C=C (the Michael reaction).
Trifluoromethyl-containing compounds 1a, 2a exhibited greater reactivity when reacting with amino-1,2,4-triazole and 2,3-diaminopyridine, since bicyclic azaheterocycles 11 and 12 were obtained in these reactions under mild conditions. C2F5-analogs 1b, 2b, however, could not be involved in such transformations (Scheme 4).
Judging by the 1H and 19F NMR spectroscopy data, the product of the reaction of amino-1,2,4-triazole with both CF3-analogs 1a and 2a is triazolopyrimidine 11. In the 1H NMR spectrum, downfield singlets recorded at 8.55 and 10.96 ppm belong to NH and OH groups, respectively. The presence of the carbonyl carbon atom signal of the acetyl group at 192.5 ppm in the 13С NMR spectrum makes it possible to make an unequivocal choice in favor of structure 11. The carbon signal at the CF3 substituent in the form of a quartet at 82.5 ppm (2JCF = 33.7 Hz) indicates its location at the quaternary carbon atom. We were unable to grow a crystal of this compound to establish its regioisomeric structure. However, literature data indicate the predominant involvement of the nitrogen atom N(2) of the starting amino-1,2,4-triazole in the cyclization.7
It was found using X-ray structural analysis that 3,4-dihydropyrido[2,3-b]pyrazine 12 is formed as a result of the reaction of CF3-furan-3(2H)-one 2a with 2,3-diaminopyridine (Fig. 1).
Based on the obtained results, it can be assumed that the initial attack of binucleophiles in the reactions of trifluoromethyl-containing compounds occurs via the keto group of the hidden α-dicarbonyl fragment, and the direction of further intramolecular cyclization is determined by the nature of the binucleophile, leading to the formation of five- or six-membered azaheterocycles. In the case of 2-methoxy-2-methyl-5-(1,1,2,2,2-pentafluoroethyl) furan-3(2H)-one, it becomes possible to realize a second reaction, 1,4-addition of N- and O-nucleophiles at the activated C=C bond (the Michael reaction). The predominant 1,2-addition of the 2-methoxy-2-methyl-5-(trifluoromethyl)furan-3(2H)-one to the enone system is due to the higher electron-withdrawing effect of the CF3 substituent as compared to the C2F5 group. In turn, the smaller influence exerted by the fluoroalkyl group upon transition to the C2F5 group in 2-methoxy-2-methyl-5-(1,1,2,2,2-pentafluoroethyl)furan-3(2H)-one reduced the regioselectivity of the processes, which we observed in the reaction with hydrazine. It may be noted that, unlike C2F5-analogs, CF3 derivatives showed higher reactivity, which allowed us to obtain new azaheterocycles under mild conditions.
Experimental
1H, 13C, and 19F NMR spectra were acquired on Bruker DRX-500 (500, 125, and 470 MHz, respectively) and Bruker DRX-400 (400, 125, and 376 MHz, respectively) spectrometers, with TMS and C6F6 as internal standards. The assignment of signals in the 13C NMR spectra are made on the basis of the spectra of analogs.5,7 Elemental analysis was performed on a PerkinElmer Series II 2400 Elemental Analyzer. Melting points were determined in open capillaries on a Stuart SMP3 apparatus. Monitoring of the reaction progress was done by TLC on Alugram Sil G/UV254 TLC plates.
The original fluorine-containing lithium diketonates 1a,b5a,e and furanone 2a5a were synthesized by known methods.
2-Methoxy-2-methyl-5-(1,1,2,2,2-pentafluoroethyl)-furan-3(2Н)-one (2b). Lithium diketonate 1b (10.00 g, 35 mmol) was dissolved in Et2O (100 ml), and a solution of oxalic acid dihydrate (12.00 g) in water (200 ml) was slowly added. The organic phase was separated, the aqueous layer was extracted with Et2O (2×50 ml). The combined organics were dried over MgSO4, the solvent was removed under reduced pressure, and the residue was distilled. Yield 5.88 g (68%), yellow liquid, bp 141–143°С (bp 143–145°C5е). 1H NMR spectrum (500 MHz, CDCl3), δ, ppm: 1.57 (3H, s, CH3); 3.31 (3H, s, CH3O); 6.11 (1H, s, CH). 13C NMR spectrum (125 MHz, CDCl3), δ, ppm (J, Hz): 20.8 (CH3); 52.8 (CH3O); 106.8 (CH3C); 108.2 (tq, 1JCF = 256.5, 2JCF = 40.4, CF2); 110.7 (CH); 117.9 (qt, 1JCF = 287.0, 2JCF = 35.3, CF3); 173.5 (t, 2JCF = 30.4, CF2C); 199.5 (C=O). 19F NMR spectrum (470 MHz, CDCl3), δ, ppm (J, Hz): 40.2 (2F, dq, 3JFF = 2.3, 2JFF = 10.0, CF2); 78.6 (3F, t, 3JFF = 2.0, CF3). Found, %: С 38.92; Н 2.71. С8Н7F5О3. Calculated, %: С 39.04; Н 2.87.
3-(1,1,2,2,2-Pentafluoroethyl)-5-[({1-[3-(1,1,2,2,2-pentafluoroethyl)-1H-pyrazol-5-yl]ethylidene}hydrazinylidene)ethyl]-1Н-pyrazole (3). Lithium diketonate 1b (0.57 g, 2 mmol) (or furanone 2b (0.49 g, 2 mmol)) and hydrazine hydrate (0.50 g, 10 mmol) were dissolved in glacial AcOH (20 ml). The mixture was heated to 100°С in a water bath for 5 h, then water (70 ml) was added. The formed precipitate was filtered off, and the product was purified by recrystallization from Et2O–hexane, 1:2 mixture. Yield 0.35 g (77%, from compound 2b), white powder, mp 234–235°C. 1H NMR spectrum (400 MHz, DMSO-d6), δ, ppm: 2.34 (6H, s, 2СН3); 7.27 (2H, s, 2CH); 14.34 (2H, s, 2NH). 13C NMR spectrum (125 MHz, DMSO-d6), δ, ppm (J, Hz): 15.2 (СН3); 101.6 (tq, 1JCF = 249.2, 2JCF = 38.8, CF2); 105.8 (C-4); 118.6 (qt, 1JCF = 285.8, 2JCF = 38.2, CF3); 140.0 (t, 2JCF = 28.5, CF2C); 142.7 (C-5); 151.1 (C=N). 19F NMR spectrum (376 MHz, DMSO-d6+ C6F6), δ, ppm: 51.7 (2F, s, CF2); 79.0 (3F, t, J = 3.8, CF3). Found, %: С 36.98; H 2.11; N 18.35. C14H10F10N6. Calculated, %: С 37.18; H 2.23; N 18.58.
5,6а-Dihydroxy-3а-methyl-5-(1,1,2,2,2-pentafluoroethyl) hexahydro-2Н-furo[2,3-d]imidazol-2-one (4). Lithium diketonate 1b (0.57 g, 2 mmol) (or furanone 2b (0.49 g, 2 mmol)) and urea (0.18 g, 3 mmol) were dissolved in glacial AcOH (15 ml); the mixture was kept at 40°С for 7 h. The formed precipitate was filtered off, washed with Et2O (2×5 ml), and dried at 70°С. Yield 0.44 g (75%, from compound 2b), white powder, mp 149–150°C. 1H NMR spectrum (400 MHz, DMSO-d6), δ, ppm: 1.30 (3H, s, СН3); 2.38 (2Н, s, CH2); 6.15 (1H, s, OH); 7.28 (1H, s, OH); 7.42 (1H, s, NH); 7.57 (1H, s, NH). 13C NMR spectrum (125 MHz, DMSO-d6), δ, ppm (J, Hz): 20.7 (СН3); 44.2 (CH2); 91.0 (CH3C); 99.1 (C–OH); 99.9 (m, CF2С); 111.6 (tq, 1JCF = 261.3, 2JCF = 34.2, CF2); 118.7 (qt, 1JCF = 287.2, 2JCF = 35.8, CF3); 157.7 (C=O). 19F NMR spectrum (376 MHz, DMSO-d6 + C6F6), δ, ppm (J, Hz): 34.1 (1F, d, 2JFF = 270.6, CFF); 38.6 (1F, d, 2JFF = 270.8, CFF); 83.8 (3F, s, CF3). Found, %: С 32.77; H 3.03; N 9.50. C8H9F5N2O4. Calculated, %: С 32.89; H 3.11; N 9.59.
3-(1-Hydroxyethanimidoyl)-5-(1,1,2,2,2-pentafluoroethyl)-4,5-dihydro-1,2-oxazol-5-ol (5). Lithium diketonate 1b (0.57 g, 2 mmol) (or furanone 2b (0.49 g, 2 mmol)) and hydroxylamine hydrochloride (0.28 g, 4 mmol) were dissolved in EtOH (10 ml); the mixture was heated under eflux for 4 h. Upon completion of the reaction, the solvent was evaporated under reduced pressure, the solid residue was washed with a mixture Et2O–hexane, 1:1, and dried at 70°С. Yield 0.45 g (84%, from compound 2b), white powder, mp 247–248°C (decomp.). 1H NMR spectrum (400 MHz, DMSO-d6), δ, ppm (J, Hz): 2.03 (3H, s, СН3); 3.28 (1Н, d, 2JНН = 18.7, CHН); 3.58 (1H, d, 2JНН = 18.5, СНН); 8.83 (1H, s, OH); 12.11 (1H, s, ОН). 13C NMR spectrum (125 MHz, DMSO-d6), δ, ppm (J, Hz): 10.1 (СН3); 41.2 (CH2); 105.6 (m, CF2С); 111.3 (tq, 1JCF = 260.4, 2JCF = 36.3, CF2); 118.5 (qt, 1JCF = 287.2, 2JCF = 35.3, CF3); 147.6 (C=NOH); 156.9 (C=N). 19F NMR spectrum (376 MHz, DMSO-d6 + C6F6), δ, ppm (J, Hz): 35.7 (1F, d, 2JFF = 274.0, CFF); 40.2 (1F, d, 2JFF = 274.0, CFF); 88.4 (3F, s, CF3). Found, %: С 31.99; H 2.57; N 10.56. C7H7F5N2O3. Calculated, %: С 32.07; H 2.69; N 10.69.
5-(1-Hydrazinylideneethyl)-3-(1,1,2,2,2-pentafluoroethyl)-1Н-pyrazole (6). Hydrazine hydrate (0.20 g, 4 mmol) was added to a solution of furanone 2b (0.50 g, 2 mmol) in MeOH (15 ml), and the mixture was heated under reflux for 2 h. The solvent was then evaporated under reduced pressure, the residue was washed with hexane and dried. Yield 0.32 g (66%), white powder, mp 127–128°C. 1H NMR spectrum (500 MHz, DMSO-d6), δ, ppm: 2.01 (3H, s, СН3); 6.66 (2H, s, NH2); 6.76 (1H, s, CH); 13.63 (1H, s, NH). 13C NMR spectrum (100 MHz, DMSO-d6), δ, ppm (J, Hz): 11.9 (СН3); 101.4 (C-4); 111.0 (tq, 1JCF = 248.7, 2JCF = 39.0, CF2); 118.7 (qt, 1JCF = 286.0, 2JCF = 38.0, CF3); 132.7 (C-5); 139.5 (t, 2JCF = 28.2, CF2С); 144.8 (C=N). 19F NMR spectrum (470 MHz, DMSO-d6 + C6F6), δ, ppm: 51.9 (2F, s, CF2); 79.0 (3F, s, CF3). Found, %: С 34.64; H 2.78; N 23.03. C7H7F5N4. Calculated, %: С 34.72; H 2.91; N 23.14.
Synthesis of substituted furans 7 and 8 (General method). Hydrazine monohydrate (0.10 g, 2 mmol) (or phenylhydrazine (2 mmol)) was added to a solution of furanone 2b (0.50 g, 2 mmol) in MeOH (15 ml) at 0–10°С. The reaction mixture was then stirred for 1 h. The formed precipitate was filtered off and washed with Et2O (2×5 ml).
[2,5-Dimethoxy-2-methyl-5-(1,1,2,2,2-pentafluoroethyl)-dihydrofuran-3(2Н)-ylidene]hydrazine (7). Yield 0.37 g (66%), white powder, mp 115–116°C. 1H NMR spectrum (400 MHz, DMSO-d6), δ, ppm (J, Hz): 1.42 (3H, s, СН3); 2.85 (1H, d, 2JHH = 18.2, СHH); 3.00 (1H, d, 2JHH = 18.5, СHH); 3.11 (3H, s, СН3О); 3.14 (3H, s, СН3О); 7.22 (1H, s, NH); 7.56 (1H, s, NH). 19F NMR spectrum (376 MHz, DMSO-d6 + C6F6), δ, ppm (J, Hz): 38.03 (1F, d, 2JFF = 268.8, CFF); 43.74 (1F, d, 2JFF = 269.0, CFF); 83.81 (3F, s, CF3). Found, %: С 36.64; H 4.25; N 9.31. C9H13F5N2O3. Calculated, %: С 36.99; H 4.48; N 9.59.
2-Methoxy-2-methyl-5-(1,1,2,2,2-pentafluoroethyl)-5-(2-phenylhydrazinyl)dihydrofuran-3(2Н)-one (8). Yield 0.51 g (84%), white powder, mp 112–113°C. 1H NMR spectrum (400 MHz, DMSO-d6), δ, ppm (J, Hz): 1.36 (3H, s, CH3); 2.82 (1H, d, 2JHH = 18.6, CHH); 3.09 (1H, d, 2JHH = 18.6, CHH); 3.38 (3H, s, СН3); 6.28 (1Н, s, NH); 6.42 (1Н, s, NH); 6.71 (1Н, t, J = 8.0, H Ph); 6.80–6.82 (2Н, m, H Ph); 7.12–7.16 (2Н, m, H Ph). 19F NMR spectrum (376 MHz, DMSO-d6 + C6F6), δ, ppm (J, Hz): 36.9 (1F, d, 2JFF = 277.2, CFF); 42.2 (1F, d, 2JFF = 277.4, CFF); 84.17 (3F, s, CF3). Found, %: С 47.21; H 4.07; N 26.62. C14H15F5N2O3. Calculated, %: С 47.46; H 4.27; N 26.81.
1-[3-(1,1,2,2,2-Pentafluoroethyl)-1H-pyrazol-5-yl]-ethanone (9). 20% Aqueous HCl (25 ml) was added to compound 7 (0.3 g, 1 mmol), the reaction mixture was heated under reflux for 2 h, the formed precipitate was then filtered off and dried. Yield 0.22 g (95%), white powder, mp 86–87°C. 1H NMR spectrum (500 MHz, DMSO-d6), δ, ppm: 2.55 (3Н, s, CH3); 7.54 (1H, s, CH); 14.73 (1H, s, NH). 13C NMR spectrum (125 MHz, DMSO-d6), δ, ppm (J, Hz): 27.3 (CH3); 108.8 (CH); 110.5 (tq, 1JCF = 249.4, 2JCF = 38.9, CF2); 118.7 (qt, 1JCF = 285.7, 2JCF = 38.3, CF3); 140.1 (t, 2JCF = 28.8, CF2C); 142.6 (C-5); 188.3 (C=O).19F NMR spectrum (470 MHz, DMSO-d6 + C6F6), δ, ppm: 51.6 (2F, s, CF2); 78.9 (3F, s, CF3). Found, %: С 36.78; H 2.14; N 12.11. C7H5F5N2O. Calculated, %: С 36.86; H 2.21; N 12.28.
Reaction of 2-methoxy-2-methyl-5-(perfluoroethyl)furan-3(2Н)-one (2b) witho-phenylenediamine. o-Phenylenediamine (0.216 g, 2 mmol) was added to a solution of furanone 2b (0.500 g, 2 mmol) in glacial AcOH (10 ml), and the mixture was heated under reflux for 3 h. After cooling to room temperature, water (50 ml) was added. The formed precipitate was filtered off, and the product was recrystallized from aqueous EtOH to afford 2-(1,1,2,2,2-pentafluoroethyl)-1H-benzimidazole (10). Yield 0.35 g (74%), white powder, mp 212–213°C (mp 210–212°C8). 1H NMR spectrum (500 MHz, DMSO-d6), δ, ppm: 7.36–7.43 (2Н, m, H Ph); 7.70–7.78 (2Н, m, H Ph); 13.96 (1Н, br. s, NH). 19F NMR spectrum (470 MHz, DMSO-d6 + C6F6), δ, ppm (J, Hz): 49.8 (2F, q, 2JFF = 2.9, CF2); 79.9 (3F, t, 2JFF = 3.0, CF3). Found, %: С 45.64; H 1.98; N 11.73. C9H5F5N2. Calculated, %: С 45.78; H 2.13; N 11.86.
1-[7-Hydroxy-7-(trifluoromethyl)-4,7-dihydro[1,2,4]-triazolo[1,5-а]pyrimidin-5-yl]ethanone (11). Lithium diketonate 1a (0.47 g, 2 mmol) (or furanone 2a (0.39 g, 2 mmol)) and amino-1,2,4-triazole (0.168 g, 2 mmol) were dissolved in glacial AcOH (10 ml), the mixture was stirred at 30–35°С for 12 h. The formed precipitate was filtered off and washed with hexane. Yield 0.36 g (58%, from compound 2a), white powder, mp 222–223°C. 1H NMR spectrum (500 MHz, DMSO-d6), δ, ppm: 2.51 (3H, s, CH3); 5.95 (1H, s, CH); 7.87 (1H, s, CH); 8.55 (1H, s, NH); 10.96 (1H, s, OH). 13C NMR spectrum (125 MHz, DMSO-d6), δ, ppm (J, Hz): 25.5 (CH3); 82.5 (q, 2JCF = 33.7, CF3С); 100.2; 122.2 (q, 1JCF = 288.0, CF3); 136.4; 148.7 (CCOCH3); 151.0; 192.5 (C=O). 19F NMR spectrum (470 MHz, DMSO-d6 + + C6F6), δ, ppm: 83.2 (3F, s, CF3). Found, %: С 38.63; H 2.69; N 22.42. C8H7F3N4O2. Calculated, %: С 38.72; H 2.84; N 22.58.
1,1,1-Trifluoro-3-(2-methylpyrido[2,3-b]pyrazin-3(4Н)-ylidene)propan-2-one (12). Furanone 2a (0.392 g, 2 mmol) and 2,3-diaminopyridine (0.218 g, 2 mmol) were dissolved in glacial AcOH (10 ml), and the mixture was stirred at room temperature for 6 h. Water (50 ml) was then added, the formed precipitate was filtered off and washed with hexane. Yield 0.53 g (82%), yellow powder, mp 189–190°C. 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 2.64 (3H, s, CH3); 5.96 (1H, s, CH); 7.46 (1H, br. s, H Ar); 8.12 (1H, d, J = 7.0, H Ar); 8.65 (1H, br. s, H Ar); 14.28 (1Н, br. s, NH). 13C NMR spectrum (125 MHz, CDCl3), δ, ppm (J, Hz): 22.3 (CH3); 85.7 (CCOCF3); 117.1 (q, 1JCF = 288.0, CF3); 122.2 (C-2(3)); 131.0 (C-3(2)); 136.3 (C-7); 140.1 (C-5); 147.2 (C-6); 151.2 (C-10); 157.0 (C-8); 178.4 (q, 2JCF = 34.4, CF3С). 19F NMR spectrum (376 MHz, CDCl3 + C6F6), δ, ppm: 85.2 (3F, s, CF3). Found, %: С 51.55; H 3.05; N 16.34. C11H8F3N3O. Calculated, %: С 51.77; H 3.16; N 16.47.
X-ray structural analysis of compound 12 was performed on an automatic 4-circle diffractometer Xcalibur 3 with CCD-detector according to the standard routine (МоKα radiation, graphite monochromator, ω-scanning with 1° step at 295(2) K). Empirical absorption correction was introduced. The structure was solved with the direct method and refined against F2 by the least-squares technique in the full-matrix anisotropic approximation for all non-hydrogen atoms. The positions of the hydrogen atoms of the CH bonds were calculated geometrically, the positions of the hydrogen atoms of the NH groups were refined independently in the isotropic approximation. All calculations were performed using the SHELXTL software set. The main crystallographic parameters of compound 12: triclinic crystals, spatial symmetry group P1; a 7.1677(10), b 11.3987(18), c 14.1021(18) Å; α 82.982(12); β 81.581(11), γ 86.054(12)°; V 1129.7(3) Å3. For substance with empirical formula C11H8F3N3O: Z 4; μ 0.134 mm–1. At angles 3.61 < θ < 26.37°, 6257 reflections were collected, 4460 (Rint 0.0565) were independent, including 1405 with I > 2σ(I). The final refinement parameters: R1 0.2056, wR2 0.2000 (over all reflections), R1 0.0702, wR2 0.1416 (over reflections with I > 2σ(I)) with the quality factor GOOF 0.997. Peaks of residual electron density 0.228/–0.249 е·Å–3. The full set of X-ray structural data for compound 12 was deposited at the Cambridge Crystallographic Data Center (deposit CCDC 1905414).
References
(a) Ananikov, V. P.; Eremin, D. B.; Yakukhnov, S. A.; Dilman, A. D.; Levin, V. V.; Egorov, M. P.; Karlov, S. S.; Kustov, L. M.; Tarasov, A. L.; Greish, A. A.; Shesterkina, A. A.; Sakharov, A. M.; Nysenko, Z. N.; Sheremetev, A. B.; Stakheev, A. Yu.; Mashkovsky, I. S.; Sukhorukov, A. Yu.; Ioffe, S. L.; Terent'ev, A. O.; Vil', V. A.; Tomilov, Y. V.; Novikov, R. A.; Zlotin, S. G.; Kucherenko, A. S.; Ustyuzhanina, N. E.; Krylov, V. B.; Tsvetkov, Y. E.; Gening, M. L.; Nifantiev, N. E. Mendeleev Commun. 2017, 27, 425. (b) Mishra, S.; Daniele, S. Chem. Rev. 2015, 115, 8379. (c) Jeffries, B.; Wang, Z.; Graton, J.; Holland, S. D.; Brind, T.; Greenwood, R. D. R.; Le Questel, J.-Y.; Scott, J. S.; Chiarparin, E.; Linclau, B. J. Med. Chem. 2018, 61, 10602. (d) Koroniak-Szejn, K.; Tomaszewska, J.; Grajewski, J.; Koroniak, H. J. Fluorine Chem. 2019, 219, 98. (e) Kaur, K.; Kumar, V.; Gupta, G. K. J. Fluorine Chem. 2015, 178, 306. (f) Kumar, H.; Saini, D.; Jain, S.; Jain, N. Eur. J. Med. Chem. 2013, 70, 248. (g) Giornal, F.; Pazenok, S.; Rodefeld, L.; Lui, N.; Vors, J.-P.; Leroux, F. R. J. Fluorine Chem. 2013, 152, 2. (h) Kuvshinova, S. S.; Smol'yakov, A. F.; Vorobyeva, D. V.; Osipov, S. N.; Loginov, D. A. Mendeleev Commun. 2018, 28, 359. (i) Bazhin, D. N.; Gorbunova, T. I.; Zapevalov, A. Ya.; Saloutin, V. I. J. Fluorine Chem. 2009, 130, 438. (j) Burgart, Ya. V.; Agafonova, N. A.; Shchegolkov, E. V.; Maslova, V. V.; Triandafilova, G. A.; Solodnikov, S. Yu.; Krasnykh, O. P.; Saloutin, V. I. Chem. Heterocycl. Compd. 2019, 55, 52. [Khim. Geterotsikl. Soedin. 2019, 55, 52.]
(a) Rulev, A. Yu.; Romanov, A. R. RSC Adv. 2016, 6, 1984. (b) Kutovaya, I. V.; Shmatova, O. I.; Nenajdenko, V. G. Mendeleev Commun. 2018, 28, 133. (c) Obydennov, D. L.; El-Tantawy, A. I.; Sosnovskikh, V. Y. J. Org. Chem. 2018, 83, 13776. (d) Muzalevskiy, V. M.; Mamedzade, M. N.; Chertkov, V. A.; Bakulev, V. A.; Nenajdenko, V. G. Mendeleev Commun. 2018, 28, 17. (e) Bazhin, D. N.; Kudyakova, Y. S.; Nemytova, N. A.; Burgart, Ya. V.; Saloutin, V. I. J. Fluorine Chem. 2016, 186, 28. (f) Kudyakova, Y. S.; Bazhin, D. N.; Slepukhin, P. A.; Burgart, Ya. V.; Saloutin, V. I.; Charushin, V. N. Tetrahedron Lett. 2017, 58, 744. (g) Palysaeva, N. V.; Boltacheva, N. S.; Slepukhin, P. A.; Pervova, M. G.; Filyakova, V. I.; Sheremetev, A. B.; Charushin, V. N. Mendeleev Commun. 2018, 28, 126. (h) Rulev, A. Yu. Eur. J. Org. Chem. 2018, 3609. (i) Mykhaylychenko, S. S.; Markitanov, Y. N.; Rudenko, T. V.; Rusanov, E. B.; Shermolovich, Y. G. Chem. Heterocycl. Compd. 2019, 55, 189. [Khim. Geterotsikl. Soedin. 2019, 55, 189.] (j) Usachev, S. А.; Tabatchikova, K. M.; Sevenard, D. V.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2018, 54, 848. [Khim. Geterotsikl. Soedin. 2018, 54, 848.] (k) Usachev, S. А.; Usachev, B. I.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2017, 53, 1294. [Khim. Geterotsikl. Soedin. 2017, 53, 1294.]
(a) Goulioukina, N. S.; Makukhin, N. N.; Beletskaya, I. P. Russ. Chem. Rev. 2016, 85, 667. [Usp. Khim. 2016, 85, 667.] (b) Korotaev, V. Y.; Kutyashev, I. B.; Barkov, A. Y.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2018, 54, 852. [Khim. Geterotsikl. Soedin. 2018, 54, 852.] (c) Sloop, J. C.; Holder, C.; Henary, M. Eur. J. Org. Chem. 2015, 3405. (d) Kudyakova, Yu. S.; Bazhin, D. N.; Goryaeva, M. V.; Burgart, Ya. V.; Saloutin, V. I. Russ. Chem. Rev. 2014, 83, 120. [Usp. Khim. 2014, 83, 120.] (e) Fustero, S.; Sánchez- Roselló, M.; Barrio, P.; Simón-Fuentes, A. Chem. Rev. 2011, 111, 6984. (f) Korotaev, V. Y.; Kutyashev, I. B.; Barkov, A. Y.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2017, 53, 597. [Khim. Geterotsikl. Soedin. 2017, 53, 597.] (g) Volkonskii, A. Yu.; Peregudov, A. S.; Strelkova, T. V.; Kagramanov, N. D. Russ. Chem. Bull., Int. Ed. 2018, 67, 164. [Izv. Akad. Nauk, Ser. Khim. 2018, 164.] (h) Usachev, S. А.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2016, 52, 1005. [Khim. Geterotsikl. Soedin. 2016, 52, 1005.] (i) Lisovenko, N. Yu.; Kozlova, E. P.; Kharitonova, S. S.; Dmitriev, М. V. Russ. J. Org. Chem. 2018, 54, 707. [Zh. Org. Khim. 2018, 54, 705.] (j) Safrygin, A. V.; Sosnovskikh, V. Ya. Russ. Chem. Rev. 2017, 86, 318. [Usp. Khim. 2017, 86, 318.] (k) Gibadullina, N. N.; Latypova, D. R.; Vakhitov, V. A.; Khasanova, D. V.; Zainullina, L. F.; Vakhitova, Y. V.; Lobov, A. N.; Ugrak, B. I.; Tomilov, Y. V.; Dokichev, V. A. J. Fluorine Chem. 2018, 211, 94. (l) Chizhov, D. L.; Belyaev, D. V.; Yachevskii, D. S.; Rusinov, G. L.; Chupakhin, O. N.; Charushin, V. N. J. Fluorine Chem. 2017, 199, 39. (m) Bazhin, D. N.; Kudyakova, Y. S.; Burgart, Ya. V.; Saloutin, V. I. Tetrahedron Lett. 2012, 53, 1961. (n) Teixeira, W. K. O.; Gonçalves, H. A.; de Mello, D. L.; Moura, S.; Flores, D. C.; Flores, A. F. C. Synth. Commun. 2017, 47, 2078. (o) Flores, A. F. C.; Malavolta, J. L.; Frigo, L. M.; Doneda, M.; Flores, D. C. Synth. Commun. 2015, 45, 1198. (p) Gonçalves, H. A.; Pereira, B. A.; Teixeira, W. K. O.; Moura, S.; Flores, D. C.; Flores, A. F. C. J. Fluorine Chem. 2016, 187, 40.
(a) Bychek, R. M.; Levterov, V. V.; Sadkova, I. V.; Tolmachev, A. A.; Mykhailiuk, P. K. Chem.–Eur. J. 2018, 24, 12291. (b) Sudarikov, D. V.; Krymskaya, Yu. V.; Il'chenko, N. O.; Slepukhin, P. A.; Rubtsova, S. A.; Kutchin, A. V. Russ. Chem. Bull., Int. Ed. 2018, 67, 731. [Izv. Akad. Nauk, Ser. Khim. 2018, 731.] (c) Ponomarenko, M. V.; Grabowsky, S.; Pal, R.; Röschenthaler, G.-V.; Fokin, A. A. J. Org. Chem. 2016, 81, 6783. (d) Iakovenko, R. O.; Dilman, A. D. Mendeleev Commun. 2018, 28, 396. (d) Haranahalli, K.; Honda, T.; Ojima, I. J. Fluorine Chem. 2019, 217, 29. (e) Nosik, P. S.; Ryabukhin, S. V.; Pashko, M. O.; Grabchuk, G. P.; Grygorenko, O. O.; Volochnyuk, D. M. J. Fluorine Chem. 2019, 217, 80. (f) Ndong, G. J. E.; Jismy, B.; Ngi, S. I.; Abarbri, M. J. Fluorine Chem. 2018, 212, 45.
(a) Bazhin, D. N.; Chizhov, D. L.; Röschenthaler, G.-V.; Kudyakova, Yu. S.; Burgart, Ya. V.; Slepukhin, P. A.; Saloutin, V. I.; Charushin, V. N. Tetrahedron Lett. 2014, 55, 5714. (b) Bazhin, D. N.; Kudyakova, Yu. S.; Röschenthaler, G.-V.; Burgart, Ya. V.; Slepukhin, P. A.; Isenov, M. L.; Saloutin, V. I.; Charushin, V. N. Eur. J. Org. Chem. 2015, 23, 5236. (c) Bazhin, D. N.; Kudyakova, Y. S.; Onoprienko, A. Y.; Slepukhin, P. A.; Burgart, Ya. V.; Saloutin, V. I. Chem. Heterocycl. Compd. 2017, 53, 1324. [Khim. Geterotsikl. Soedin. 2017, 53, 1324.] (d) Bazhin, D. N.; Kudyakova, Yu. S.; Slepukhin, P. A.; Burgart, Ya. V.; Malysheva, N. M.; Kozitsina, A. N.; Ivanova, A. V.; Bogomyakov, A. S.; Saloutin, V. I. Mendeleev Commun. 2018, 28, 202. (e) Bazhin, D. N.; Kudyakova, Y. S.; Burgart, Ya. V.; Saloutin, V. I. Russ. Chem. Bull., Int. Ed. 2018, 67, 497. [Izv. Akad. Nauk, Ser. Khim. 2018, 497.] (f) Krisyuk, V. V.; Kyzy, S. U.; Rybalova, T. V.; Baidina, I. A.; Korolkov, I. V.; Chizhov, D. L.; Bazhin, D. N.; Kudyakova, Y. S. J. Coord. Chem. 2018, 71, 2194. (g) Bazhin, D. N.; Kudyakova, Yu. S.; Bogomyakov, A. S.; Slepukhin, P. A.; Kim, G. A.; Burgart, Ya. V.; Saloutin, V. I. Inorg. Chem. Front. 2019, 6, 40.
(a) Safrygin, A. V.; Irgashev, R. A.; Slepukhin, P. A.; Röschenthaler, G.-V.; Sosnovskikh, V. Ya. Tetrahedron 2015, 71, 8535. (b) Sosnovskikh, V. Ya.; Safrygin, A. V.; Irgashev, R. A.; Ezhikova, M. A.; Kodess, M. I. RSC Adv. 2016, 36, 30056.
(a) Pryadeina, M. V.; Burgart, Ya. V.; Saloutin, V. I.; Chupakhin, O. N. Mendeleev Commun. 2008, 18, 276. (b) Gol, R. M.; Khatri, T. T.; Barot, V. M. Chem. Heterocycl. Compd. 2019, 55, 246. [Khim. Geterotsikl. Soedin. 2019, 55, 246.]
Saloutina, L. V.; Zapevalov, A. Ya.; Saloutin, V. I.; Kodess, M. I.; Kirichenko, V. E.; Pervova, M. G.; Chupakhin, O. N. J. Fluorine Chem. 2005, 126, 976.
This work was financially supported by the Russian Foundation for Basic Research (project No. 18-33-20124).
Yu. S. Kudyakova is grateful to the Grants Council of the President of the Russian Federation for financial support (grant 1453.2019.3).
Registration of NMR spectra, elemental analysis, and X-ray diffraction studies were carried out on the equipment of the Center for Collective Use “Spectroscopy and analysis of organic compounds” at I. Ya. Postovsky Institute of Organic Synthesis, Ural Branch of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information file containing 1Н, 13С, and 19F NMR spectra of all synthesized compounds is available at the journal website at http://springerlink.bibliotecabuap.elogim.com/journal/10593.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(6), 517–522
Electronic supplementary material
ESM 1
(PDF 1160 kb)
Rights and permissions
About this article
Cite this article
Kudyakova, Y.S., Onoprienko, A.Y., Slepukhin, P.A. et al. Fluorine-Containing Furan-3(2Н)-Ones in Reactions with Binucleophiles: CF3vs C2F5. Chem Heterocycl Comp 55, 517–522 (2019). https://doi.org/10.1007/s10593-019-02488-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02488-4