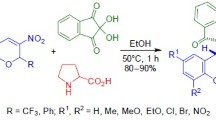
1,3-Dipolar cycloaddition of unstabilized azomethine ylide obtained in situ from sarcosine and paraform occurred stereoselectively at the double bond of 3-nitro-2-phenyl-2-(trifluoromethyl)-2H-chromenes activated by the presence of nitro group. The synthetic procedure required refluxing in benzene for 2 h and gave chromeno[3,4-c]pyrrolidines with trans configuration of trifluoromethyl group and nitro group as the major products. The structure of the obtained products was confirmed by X-ray structural analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The structural motif of pyrano[3,4-c]pyrrolidine represents a key feature of many biologically active molecules.1 Of particular interest are derivatives of chromeno[3,4-c]-pyrrolidine, some of which have been recognized as promising pharmaceutical agents.2 For example, fiduxosin shows α1A/α1B selectivity with respect to adrenoceptors and has been proposed as an improved analog of tamsulozine for the treatment of benign prostatic hyperplasia,2a,b while the investigational drug S33138 is able to block the dopamine receptor D3 and can be used in the therapy of cognitive dysfunctions, schizophrenia, and Parkinson's disease (Fig. 1).2c,d
Despite the pronounced biological activity, chromeno-[3,4-c]pyrrolidines in general have not yet been thoroughly studied and there are relatively few methods available for their synthesis, which rely on stereoselective nucleophilic addition and cycloaddition reactions.3 A simple and effective method for the synthesis of cis-fused chromeno-[3,4-c]pyrrolidines, which has recently attracted significant attention, is based on 1,3-dipolar cycloaddition of azomethine ylides at C=C bond of 3-nitro-2H-chromenes, which is activated by the presence of nitro group.4 It should be noted that when the starting nitrochromene contains only one substituent at the position C-2, the 1,3-dipole preferentially attacks the double bond from the side of the less bulky 2-CH hydrogen atom. Thus, the addition of azomethine ylide generated in situ from paraformaldehyde and sarcosine to 2-aryl- and 2-trihalomethyl-substituted 3-nitro-2H-chromenes 1 resulted in the formation of chromeno[3,4-c]pyrrolidines 2 and 3 with cis configuration of the substituent R (R = Ar, CX3) and nitro group in the pyran ring (Scheme 1).5 In this context, we were interested in characterizing the reactivity of 2,2-disubstituted 3-nitro-2H-chromenes bearing various substituents at the С-2 atom.

Scheme 1
We recently reported the preparation of 2-phenyl-2-trifluoromethyl-2H-chromenes 46a that represent structural hybrids of 2-Ph- and 2-CF3-2H-chromenes 1, as well as studied their reactions with enamines6a and sodium azide.6b In a continuation of studies focused on the effects of 2-trihalomethyl group on the reactivity of 3-nitro-2Hchromenes and the stereoselectivity of processes involving such compounds,7 in the currect work, we explored the [3+2] cycloaddition of azomethine ylides obtained from formaldehyde and sarcosine to 3-nitro-2H-chromenes 4 and developed a stereoselective method for the synthesis of 3a,4,4-trisubstituted chromeno[3,4-с]pyrrolidines.
Since the most stable configuration of chromenes 4 featured axial orientation of the phenyl substituent,6a it could be expected that the attack by azomethine ylide on the double bond from the side of equatorial CF3 group would be more favorable. Indeed, the reaction of nitrochromenes 4a–f with ylide derived from formaldehyde and sarcosine, performed by refluxing in benzene for 2 h, gave 92–99% yields of mixtures containing chromeno[3,4-c]-pyrrolidines trans-5a–f and cis-5a–f, with the major diastereomer having trans configuration of the nitro group and CF3 group in the pyran ring (Scheme 2, Table 1).

Scheme 2
As shown in Table 1, the yields of products 5a–f did not depend on the electron donor or acceptor properties of the substituents R1 and R2, but the fraction of the minor cis-isomer did not exceed 30–33%.
1H NMR spectra of mixtures containing diastereomeric adducts 5a–f were acquired in DMSO-d6 solutions and showed the characteristic doublet signals of 3-CH2 methylene protons in the range of 4.05−4.79 ppm with spin-spin coupling constants 2J = 11.2–12.1 Hz and the triplet signal of 9b-СН benzyl proton was observed at 4.05–4.79 ppm with spin-spin coupling constant of 2J = 6.7–7.9 Hz. 13C NMR spectra of both the trans- and cis-isomers of compounds 5a–f contained quartet signals of CF3 group and С-4 atom in the ranges of 122.9–124.0 and 78.2–80.8 ppm, respectively, with spin-spin coupling constants 1JCF = 289.1–292.6 and 2JCF = 27.6–28.5 Hz. IR spectra of products 5a–f featured characteristic nitro group vibration bands ν(NO2) in the intervals of 1549−1557 and 1337−1342 cm−1.
The reaction of pyrrolidines 5a–f with oxalic acid in 8:3 hexane−Me2CO mixture gave high yields (67–84%) of the respective oxalates 6a–f. As a result of this treatment, the content of cis-isomers in oxalates 6c,d dropped to 24 and 19%, respectively, while 6,8-dihalo-substituted derivatives 6e,f were practically completely purified from the presence of cis-adduct (Scheme 3, Table 2).

Scheme 3
The individual trans-isomers 5a–f were obtained by washing oxalates 6a–f with acetone, suspending in CH2Cl2, and then adding aqueous NaOH solution (Scheme 4, Table 3).

Scheme 4
The spatial structure of the major trans-isomers 5a–f was confirmed by monocrystal X-ray diffraction analysis of compound trans-5d (Fig. 2). Cycloadduct trans-5d was indeed the isomer containing nitro group and trifluoromethyl group in a transoid relationship, with the latter occupying an axial position. The pyran and pyrrolidine rings were fused in a cis-3a,9b-equatorially-axial geometry and the rings had a distorted half chair and twist conformations, respectively.
The same pyran ring conformation, but with trans configuration of CF3 group relative to the pyrrolidine moiety apparently was present also in compounds cis-5a–f (Fig. 3). The axial orientation of trifluoromethyl substituent in both diastereomers was indicated by the close chemical shift values for the CF3 groups in 19F NMR spectra of trans- and cis-adducts 5a–f, acquired in DMSO-d6 solution (94.7−95.3 ppm and 95.1−95.6 ppm, respectively). The pseudoequatorial 9b-CH proton in the case of transisomers 5a–f was observed as a triplet in the range of 4.05−4.23 ppm, while in cis-isomers 5a–f this proton was in a pseudoaxial orientation and was shifted downfield due to the deshielding effect of the CF3 group (4.64−4.79 ppm). The close proximity of CF3 group and the С-3 atom of pyrrolidine ring in compounds trans-5a–f caused the latter 13С NMR signal to appear as a quartet at 60.4−61.1 ppm with spin-spin coupling constant JCF = 2.0−2.4 Hz. Such spin-spin coupling through space was absent in the cis-isomers. We should also note the greater chemical nonequivalence of 3-CH2 methylene protons in the trans-isomers 5a–f (Δδ 1.15−1.22 ppm) compared to the cis-isomers (Δδ 0.38−0.54 ppm). The same trends were observed in 1H, 19F, and 13С NMR spectra of stereoisomeric oxalates 6a–f.
Unfortunately, the reaction of nitrochromenes 4a–f with ylide obtained from formaldehyde and proline proceeded nonselectively and led to the formation of mixtures containing approximately equal amounts of four regio- and stereoisomeric products, which we were unable to separate during this study.
Thus, the azomethine ylide obtained from formaldehyde and sarcosine was used in cycloaddition reactions with 3-nitro-2-phenyl-2-(trifluoromethyl)-2H-chromenes, which predominantly proceeded as an attack from the side of equatorial trifluoromethyl group and led to the formation of chromeno[3,4-c]pyrrolidines with a trans configuration of NO2 and CF3 groups as the major products. The different solubility of oxalates derived from stereoisomeric adducts in acetone allowed to purify the major isomer from the presence of the minor cis-isomer.
Experimental
IR spectra were recorded on a Bruker Alpha spectrometer equipped with an ATR accessory (ZnSe crystal). 1Н and 19F NMR spectra were acquired on a Bruker DRX-400 spectrometer (400 and 376 MHz, respectively) in DMSO-d6, using TMS and C6F6 as internal standards. 13C NMR spectra were acquired on a Bruker Avance 500 spectrometer (126 MHz) in DMSO-d6, using the solvent signal as internal standard (40.0 ppm). High- resolution mass spectra (electrospray ionization) were recorded on a Waters Xevo Qtof instrument. Elemental analysis was performed on a PE 2400 automatic analyzer. Melting points were determined on an SMP40 apparatus.
The starting nitrochromenes 4a–f were obtained according to a published procedure.6a
Synthesis of chromeno[3,4- c ]pyrrolidines 5a–f (General method). A mixture of the appropriate nitrochromene 4a–f (1.0 mmol), paraform (0.18 g, 6.0 mmol), and sarcosine (0.22 g, 2.5 mmol) in benzene (5 ml) was stirred and refluxed in a flask with Dean–Stark trap for 2 h. The reaction mixture was then cooled to room temperature, the unreacted residue of sarcosine was removed by filtration. Solvent was removed from the filtrate at reduced pressure and the residue containing a mixture of the product stereoisomers was washed with H2O and dried at reduced pressure and room temperature. Chromenopyrrolidines 5a–f were obtained as a mixture of trans- and cis-isomers.
(3a S *,4 R *,9b R *)-2-Methyl-3a-nitro-4-phenyl-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4- c ]pyrrole ( trans- 5a) and (3a S *,4 S *,9b R *)-2-methyl-3a-nitro4-phenyl-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4- c ]pyrrole ( cis- 5a), 67:33 mixture of trans:cis isomers. Yield 0.36 g (95%), light-yellow oil. IR spectrum, ν, cm−1: 1557, 1494, 1455, 1365, 1342. 1H NMR spectrum, δ, ppm (J, Hz): trans-isomer: 2.29 (1H, t, J = 8.1, 1-CHH); 2.34 (3H, s, NCH3); 3.09 (1H, d, J = 12.0, 3-СHH); 3.67 (1H, t, J = 8.7, 1-СHH); 4.10 (1H, t, J = 7.5, 9b-CH); 4.27 (1H, d, J = 12.0, 3-СHH); 7.11−7.27 (4H, m, H Ar); 7.39−7.51 (5H, m, H Ph); cis-isomer: 2.09 (3H, s, NCH3); 2.27 (1H, dd, J = 9.2, J = 5.3, 1-CHH); 2.66 (1H, d, J = 11.2, 3-СHH); 3.04 (1H, d, J = 11.2, 3-СHH); 4.71 (1H, t, J = 6.7, 9b-CH); 7.27−7.37 (4H, m, H Ar); 7.52−7.69 (5H, m, H Ph); the signal of 1-СHH proton overlapped with the signal of 1-CHH proton in the transisomer. 13C NMR spectrum, δ, ppm (J, Hz): trans-isomer: 40.8 (NCH3); 43.2 (C-9b); 60.5 (q, J = 2.3, C-3); 60.6 (C-1); 79.7 (q, J = 27.8, C-4); 98.4; 116.9; 123.2 (q, J = 289.8, CF3); 123.7; 126.9 (2C Ph); 127.7; 128.2; 128.3 (2C Ph); 128.4; 129.9; 131.2; 148.6; cis-isomer: 40.4 (NCH3); 41.2 (C-9b); 62.0; 63.2; 78.2 (q, J = 27.7, C-4); 97.4; 116.5; 123.5 (q, J = 292.3, CF3); 123.5; 126.7 (q, J = 2.0, 2C Ph); 127.8; 128.1; 128.2; 128.3 (2C Ph); 130.0; 132.2; 148.4. 19F NMR spectrum, δ, ppm: trans-isomer: 95.1 (s, CF3); cis-isomer: 95.0 (s, CF3). Found, m/z: 379.1263 [М+Н]+. C19H18F3N2O3. Calculated, m/z: 379.1264.
(3a S *,4 R *,9b R *)-2,8-Dimethyl-3a-nitro-4-phenyl-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4- c ]pyrrole ( trans- 5b) and (3a S *,4 S *,9b R *)-2,8-dimethyl3a-nitro-4-phenyl-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4- c ]pyrrole ( cis- 5b), 68:32 mixture of trans- and cis-isomers. Yield 0.38 g (98%), light-yellow oil. IR spectrum, ν, cm−1: 1555, 1499, 1454, 1338. 1H NMR spectrum, δ, ppm (J, Hz): trans-isomer: 2.26 (3H, s, CH3); 2.34 (3H, s, NCH3); 2.28 (1H, t, J = 8.2, 1-CHH); 3.07 (1H, d, J = 12.0, 3-СHH); 3.64 (1H, t, J = 8.5, 1-СHH); 4.05 (1H, t, J = 7.5, 9b-CH); 4.25 (1H, d, J = 12.0, 3-СHH); 7.01−7.16 (3H, m, H-6,7,9); 7.38−7.49 (5H, m, H Ph); cis-isomer: 2.09 (3H, s, NCH3); 2.29 (3H, s, CH3); 2.26 (1H, dd, J = 9.2, J = 5.3, 1-CHH); 2.62 (1H, d, J = 11.2, 3-СHH); 3.04 (1H, d, J = 11.2, 3-СHH); 4.64 (1H, t, J = 6.7, 9b-CH); 7.01−7.16 (3H, m, H-6,7,9); 7.51−7.66 (5H, m, H Ph); the signal of 1-СHH proton overlapped with the signal of 1-CHH proton in the transisomer. 13C NMR spectrum, δ, ppm (J, Hz): trans-isomer: 20.2 (CH3); 40.8 (NCH3); 43.2 (C-9b); 60.5 (q, J = 2.2, C-3); 60.6 (C-1); 79.7 (q, J = 27.8, C-4); 98.5; 116.7; 123.2 (q, J = 289.8, CF3); 123.3; 126.9 (2C Ph); 127.8; 128.2; 128.3 (2C Ph); 129.0; 129.8; 132.7; 146.5; cis-isomer: 20.2 (CH3); 40.4 (NCH3); 41.3 (C-9b); 62.0; 63.2; 78.2 (q, J = 27.7, C-4); 97.6; 116.2; 123.5 (q, J = 292.3, CF3); 126.7 (q, J = 1.9, 2C Ph); 127.9; 128.2; 128.3 (2C Ph); 129.9; 131.2; 132.3; 132.7; 146.3. 19F NMR spectrum, δ, ppm: trans-isomer: 95.1 (s, CF3); cis-isomer: 95.2 (s, CF3). Found, m/z: 393.1419 [М+Н]+. C20H20F3N2O3. Calculated, m/z: 393.1421.
(3a S *,4 R *,9b R *)-8-Methoxy-2-methyl-3a-nitro-4-phenyl-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4- c ]pyrrole ( trans- 5c) and (3a S *,4 S *,9b R *)8-methoxy-2-methyl-3a-nitro-4-(trifluoromethyl)4-phenyl-1,2,3,3a,4,9b-hexahydrochromeno[3,4- c ]pyrrole ( cis- 5c), 69:31 mixture of trans- and cis-isomers. Yield 0.39 g (96%), light-yellow oil. IR spectrum, ν, cm−1: 1555, 1499, 1450, 1341. 1H NMR spectrum, δ, ppm (J, Hz): trans-isomer: 2.34 (3H, s, NCH3); 2.29 (1H, t, J = 8.1, 1-CHH); 3.06 (1H, d, J = 11.8, 3-СHH); 3.67 (1H, t, J = 8.5, 1-СHH); 4.07 (1H, t, J = 7.5, 9b-CH); 4.26 (1H, d, J = 11.8, 3-СHH); 6.78 (1H, d, J = 3.0, H-9); 6.89 (1H, dd, J = 8.9, J = 3.0, H-7); 7.12 (1H, d, J = 8.9, H-6); 7.38−7.50 (5H, m, H Ph); cis-isomer: 2.09 (3H, s, NCH3); 2.23 (1H, dd, J = 9.2, J = 5.8, 1-CHH); 2.59 (1H, d, J = 11.2, 3-СHH); 3.63 (1H, dd, J = 9.2, J = 8.1, 1-CHH); 4.66 (1H, t, J = 7.0, 9b-CH); 6.85 (1H, dd, J = 8.9, J = 3.0, H-7); 6.94 (1H, d, J = 3.0, H-9); 7.08 (1H, d, J = 8.9, H-6); 7.51−7.67 (5H, m, H Ph); the signal of 3-СHH proton overlapped with the signal of 3-CHH proton in the transisomer. 13C NMR spectrum, δ, ppm (J, Hz): trans-isomer: 41.3 (NCH3); 44.2 (C-9b); 55.8 (CH3O); 61.0 (C-1); 61.1 (q, J = 2.3, C-3); 80.3 (q, J = 27.6, C-4); 99.0; 112.5; 114.8; 118.3; 123.7 (q, J = 290.0, CF3); 127.5 (2C Ph); 128.7 (2C Ph); 128.8; 130.3; 131.8; 142.8; 155.7; cisisomer: 40.9 (NCH3); 42.4 (C-9b); 55.9 (CH3O); 62.7; 63.8; 78.8 (q, J = 27.7, C-4); 98.3; 112.6; 114.5; 117.9; 124.0 (q, J = 292.6, CF3); 127.2 (q, J = 1.6, 2C Ph); 128.7 (2C Ph); 128.8, 130.4; 132.8; 142.6; 155.8. 19F NMR spectrum, δ, ppm: trans-isomer: 95.2 (s, CF3); cis-isomer: 95.3 (s, CF3). Found, m/z: 409.1370 [М+Н]+. C20H20F3N2O4. Calculated, m/z: 409.1370.
(3a S *,4 R *,9b R *)-6-Ethoxy-2-methyl-3a-nitro-4-phenyl4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4- c ]pyrrole ( trans- 5d) and (3a S *,4 S *,9b R *)-6-ethoxy-2-methyl3a-nitro-4-phenyl-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4- c ]pyrrole ( cis- 5d), 70:30 mixture of trans- and cis-isomers. Yield 0.42 g (99%), colorless prisms, mp 135–136°C. IR spectrum, ν, cm−1: 1549, 1474, 1337. 1H NMR spectrum, δ, ppm (J, Hz): trans-isomer: 1.39 (3H, t, J = 7.0, OCH2CH3); 2.29 (1H, t, J = 8.0, 1-CHH); 2.32 (3H, s, NCH3); 3.08 (1H, d, J = 11.8, 3-СHH); 3.65 (1H, t, J = 8.4, 1-СHH); 4.16 (1H, t, J = 7.4, 9b-CH); 4.16 (1H, dq, J = 9.9, J = 7.0, OCHHCH3); 4.18 (1H, dq, J = 9.9, J = 7.0, OCHHCH3); 4.23 (1H, d, J = 11.8, 3-СHH); 6.72−6.76 (1H, m, H-7); 6.98−7.04 (2H, m, H-8,9); 7.37−7.45 (5H, m, H Ph); cis-isomer: 1.31 (3H, t, J = 6.9, CH3); 2.19 (1H, dd, J = 9.2, J = 5.9, 1-CHH); 2.09 (3H, s, NCH3); 2.57 (1H, d, J = 11.3, 3-СHH); 3.11 (1H, d, J = 11.3, 3-СHH); 3.61 (1H, t, J = 8.6, 1-CHH); 4.09 (1H, dq, J = 10.0, J = 7.0, OCHHCH3); 4.14 (1H, dq, J = 10.0, J = 7.0, OCHHCH3); 4.66 (1H, t, J = 6.8, 9b-CH); 6.87−6.90 (1H, m, H-7); 6.96−7.08 (2H, m, H-8,9); 7.52−7.70 (5H, m, H Ph). 13C NMR spectrum, δ, ppm (J, Hz): transisomer: 14.6 (CH3); 40.8 (NCH3); 43.1 (C-9b); 60.7 (C-1); 60.9 (q, J = 2.4, C-3); 64.1 (OCH2); 79.8 (q, J = 28.1, C-4); 98.3; 112.1; 118.8; 123.2 (q, J = 289.1, CF3); 123.6; 125.0; 126.8 (2C Ph); 128.3 (2C Ph); 129.8; 131.3; 138.0; 147.4; cis-isomer: 14.6 (CH3); 40.4 (NCH3); 41.8 (C-9b); 62.2; 63.3; 64.3 (OCH2); 78.2 (q, J = 28.0, C-4); 97.8; 112.5; 118.9; 123.4 (q, J = 292.2, CF3); 123.5; 126.0; 126.7 (q, J = 1.5, 2C Ph); 128.2 (2C Ph); 129.9; 132.4; 138.0; 147.3. 19F NMR spectrum, δ, ppm: trans-isomer: 94.5 (s, CF3); cisisomer: 95.0 (s, CF3). Found, m/z: 423.1523 [М+Н]+. C21H22F3N2O4. Calculated, m/z: 423.1526.
(3a S *,4 R *,9b R *)-6,8-Dichloro-2-methyl-3a-nitro-4-phenyl4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4- c ]pyrrole ( trans- 5e) and (3a S *,4 S *,9b R *)-6,8-dichloro-2-methyl3a-nitro-4-phenyl-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4- c ]pyrrole ( cis- 5e), 69:31 mixture of trans- and cis-isomers. Yield 0.41 g (92%), white powder, mp 161–162°C. IR spectrum, ν, cm−1: 1550, 1454, 1340. 1H NMR spectrum, δ, ppm (J, Hz): trans-isomer: 2.30 (1H, t, J = 8.5, 1-CHH); 2.37 (3H, s, NCH3); 3.13 (1H, d, J = 12.1, 3-СHH); 3.70 (1H, t, J = 8.4, 1-СHH); 4.22 (1H, t, J = 7.9, 9b-CH); 4.33 (1H, d, J = 12.1, 3-СHH); 7.40 (1H, dd, J = 2.3, J = 1.0, H-9); 7.45−7.56 (5H, m, H Ph); 7.71 (1H, d, J = 2.3, H-6); cis-isomer: 2.10 (3H, s, NCH3); 2.22 (1H, dd, J = 9.2, J = 6.1, 1-CHH); 2.64 (1H, d, J = 11.4, 3-СHH); 3.12 (1H, d, J = 11.4, 3-СHH); 3.62 (1H, t, J = 8.7, 1-СHH); 4.78 (1H, t, J = 7.0, 9b-CH); 7.53−7.70 (9H, m, H-6,9, H Ph). 13C NMR spectrum, δ, ppm (J, Hz): trans-isomer: 40.6 (NCH3); 44.0 (C-9b); 60.2 (C-1); 60.4 (q, J = 2.0, C-3); 80.6 (q, J = 28.1, C-4); 98.0; 122.5; 122.9 (q, J = 290.0, CF3); 126.3; 126.6 (2C Ph); 127.6; 128.2; 128.5; 128.6 (2C Ph); 130.3; 130.5; 143.7; cis-isomer: 40.1 (NCH3); 41.9 (C-9b); 62.2; 63.0; 79.1 (q, J = 28.5, C-4); 97.4; 122.1; 123.2 (q, J = 291.9, CF3); 126.4; 126.6 (2C Ph); 127.1; 128.0, 128.2; 128.6 (2C Ph); 130.3; 131.4; 143.4. 19F NMR spectrum, δ, ppm: transisomer: 95.4 (s, CF3); cis-isomer: 94.7 (s, CF3). Found, m/z: 447.0483 [М+Н]+. C19H16Cl2F3N2O3. Calculated, m/z: 447.0485.
(3a S *,4 R *,9b R *)-6,8-Dibromo-2-methyl-3a-nitro4-phenyl-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4- c ]pyrrole ( trans- 5f) and (3a S *,4 S *,9b R *)6,8-dibromo-2-methyl-3a-nitro-4-phenyl-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4- c ]pyrrole ( cis- 5f), 69:31 mixture of trans- and cis-isomers. Yield 0.50 g (93%), white powder, mp 151–152°C. IR spectrum, ν, cm−1: 1553, 1448, 1341. 1H NMR spectrum, δ, ppm (J, Hz): transisomer: 2.29 (1H, t, J = 8.5, 1-CHH); 2.37 (3H, s, NCH3); 3.12 (1H, d, J = 12.1, 3-СHH); 3.71 (1H, t, J = 8.4, 1-СHH); 4.23 (1H, t, J = 7.9, 9b-CH); 4.34 (1H, d, J = 12.2, 3-СHH); 7.45−7.60 (6H, m, H Ph, H-9); 7.91 (1H, d, J = 2.3, H-6); cis-isomer: 2.10 (3H, s, NCH3); 2.20 (1H, dd, J = 9.3, J = 6.1, 1-CHH); 2.62 (1H, d, J = 11.5, 3-СHH); 3.13 (1H, d, J = 11.5, 3-СHH); 3.61 (1H, t, J = 8.7, 1-СHH); 4.79 (1H, t, J = 7.1, 9b-CH); 7.55−7.72 (9H, m, H-9, H Ph); 7.87 (1H, d, J = 2.2, H-6). 13C NMR spectrum, δ, ppm (J, Hz): trans-isomer: 40.6 (NCH3); 44.0 (C-9b); 60.2 (C-1); 60.4 (q, J = 2.0); 80.8 (q, J = 28.1, C-4); 98.1; 111.9; 115.6; 122.9 (q, J = 290.3, CF3); 126.7 (2C); 127.3; 128.6 (2C); 129.7; 130.3; 130.6; 133.5; 145.1; cis-isomer: 40.1 (NCH3); 42.0 (C-9b); 62.2; 63.1; 79.2 (q, J = 28.1, C-4); 97.5; 111.4; 115.6; 123.1 (q, J = 291.8, CF3); 126.6 (q, J = 1.5, 2C Ph); 128.2; 128.5 (2C Ph); 129.8; 130.2; 131.4; 133.3; 144.8. 19F NMR spectrum, δ, ppm: trans-isomer: 95.6 (s, CF3); cis-isomer: 94.8 (s, CF3). Found, m/z: 534.9471 [М+Н]+. C19H16Br2F3N2O3. Calculated, m/z: 534.9474.
Synthesis of chromeno[3,4- c ]pyrrolidine oxalates 6a–f. The appropriate mixture of diastereomeric chromeno[3,4-c]pyrrolidines 5a–f was dissolved in an 8:3 mixture of hexane and acetone (9 ml). The obtained solution was stirred and treated by dropwise addition of oxalic acid (0.11 g, 1.2 mmol) solution in anhydrous acetone (2 ml). The reaction mixture was maintained for 30 min. The obtained precipitate was filtered off, washed with H2O (5×1 ml), and dried at 100°C. Products 6a–f were isolated as white powders.
(3a S *,4 R *,9b R *)-2-Methyl-3a-nitro-4-phenyl-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4- c ]pyrrole oxalate ( trans- 6a) and (3a S *,4 S *,9b R *)-2-methyl3a-nitro-4-phenyl-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno-[3,4- c ]pyrrole oxalate ( cis- 6a), 69:31 mixture of trans- and cis-isomers. Yield 0.39 g (84%), mp 209−210°C (decomp.). IR spectrum, ν, cm−1: 1751, 1651, 1560, 1492, 1455, 1361, 1336. 1H NMR spectrum, δ, ppm (J, Hz): trans-isomer: 2.35 (1H, t, J = 8.0, 1-CHH); 2.37 (3H, s, NCH3); 3.14 (1H, d, J = 12.0, 3-СHH); 3.68 (1H, t, J = 8.4, 1-СHH); 4.12 (1H, t, J = 7.4, 9b-CH); 4.30 (1H, d, J = 12.0, 3-СHH); 7.10−7.36 (4H, m, H Ar); 7.38−7.51 (5H, m, H Ph); cis-isomer: 2.13 (3H, s, NCH3); 2.73 (1H, d, J = 11.4, 3-СHH); 3.10 (1H, d, J = 11.4, 3-СHH); 3.65 (1H, t, J = 8.6, 1-CHH); 4.72 (1H, t, J = 6.7, 9b-CH); 7.10−7.69 (9H, m, H Ar, H Ph); the signal of 1-СHH proton in the cis-isomer overlapped with the signal of NCH3 protons in the trans-isomer. 13C NMR spectrum, δ, ppm (J, Hz): trans-isomer: 40.9 (NCH3); 43.3 (C-9b); 60.4 (q, J = 2.4, C-3); 60.5 (C-1); 79.7 (q, J = 27.9, C-4); 98.3; 117.0; 123.2 (q, J = 289.7, CF3); 123.8; 127.0 (2C Ph); 127.8; 128.3; 128.4 (2C Ph); 128.5; 130.0; 131.2; 148.7; 161.2 (2C CO2H); cis-isomer: 40.6 (NCH3); 41.3 (C-9b); 61.9; 63.1; 78.2 (q, J = 27.6, C-4); 97.4; 116.6; 123.4 (q, J = 292.2, CF3); 123.4; 124.7; 126.8 (q, J = 1.8, 2C Ph); 127.9; 128.2; 128.4 (2C Ph); 130.1; 132.1; 148.5; 161.2 (2C CO2H). 19F NMR spectrum, δ, ppm: trans-isomer: 95.1 (s, CF3); cis-isomer: 95.0 (s, CF3). Found, %: С 53.77; Н 4.24; N 6.05. C19H17F3N2O3·(CO2H)2. Calculated, %: С 53.85; Н 4.09; N 5.98.
(3a S *,4 R *,9b R *)-2,8-Dimethyl-3a-nitro-4-phenyl-4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4- c ]pyrrole oxalate ( trans- 6b) and (3a S *,4 S *,9b R *)-2,8-dimethyl-3a-nitro-4-phenyl-4-(trifluoromethyl)-1,2,3,3a,4,9bhexahydrochromeno[3,4- c ]pyrrole oxalate ( cis- 6b), 68:32 mixture of trans- and cis-isomers. Yield 0.40 g (84%), mp 214– 215°C (decomp.). IR spectrum, ν, cm−1: 1730, 1634, 1563, 1502, 1451, 1365, 1328. 1H NMR spectrum, δ, ppm (J, Hz): trans-isomer: 2.26 (3H, s, CH3); 2.33 (1H, t, J = 7.9, 1-CHH); 2.35 (3H, s, NCH3); 3.11 (1H, d, J = 11.8, 3-СHH); 3.66 (1H, t, J = 8.6, 1-СHH); 4.06 (1H, t, J = 7.4, 9b-CH); 4.28 (1H, d, J = 11.8, 3-СHH); 7.00−7.17 (3H, m, H-6,7,9); 7.38−7.49 (5H, m, H Ph); cis-isomer: 2.12 (3H, s, CH3); 2.29 (3H, s, NCH3); 2.67 (1H, d, J = 11.4, 3-СHH); 3.08 (1H, d, J = 11.4, 3-СHH); 3.62 (1H, t, J = 9.2, 1-CHH); 4.65 (1H, t, J = 6.7, 9b-CH); 7.00−7.17 (3H, m, H-6,7,9); 7.51−7.67 (5H, m, H Ph); the signal of 1-СHH proton of the cis-isomer overlapped with the signals of trans-isomer. 13C NMR spectrum, δ, ppm (J, Hz): trans-isomer: 20.2 (8-CH3); 41.0 (NCH3); 43.2 (C-9b); 60.3 (q, J = 2.3, C-3); 60.4 (C-1); 79.7 (q, J = 27.8, C-4); 98.2; 116.4; 122.8; 123.2 (q, J = 289.5, CF3); 127.0 (2C Ph); 127.9; 128.4 (2C Ph); 129.2; 130.0; 131.1; 132.8; 146.5; 161.2 (2C CO2H); cis-isomer: 20.3 (8-CH3); 40.7 (NCH3); 41.5 (C-9b); 61.6; 62.9; 78.1 (q, J = 28.2, C-4); 97.5; 116.8; 123.5 (q, J = 292.4, CF3); 123.9; 126.7 (q, J = 1.6, 2C Ph); 128.0; 128.4 (2C Ph); 129.0; 130.1; 131.1; 132.0; 146.4; 161.2 (2C CO2H). 19F NMR spectrum, δ, ppm: trans-isomer: 95.0 (s, CF3); cis-isomer: 95.1 (s, CF3). Found, %: С 54.57; Н 4.44; N 5.84. C20H19F3N2O3·(CO2H)2. Calculated, %: С 54.78; Н 4.39; N 5.81.
(3a S *,4 R *,9b R *)-8-Methoxy-2-methyl-3a-nitro-4-phenyl4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4- c ]pyrrole oxalate ( trans- 6c) and (3a S *,4 S *,9b R *)-8-methoxy-2-methyl-3a-nitro-4-phenyl-4-(trifluoromethyl)1,2,3,3a,4,9b-hexahydrochromeno[3,4- c ]pyrrole oxalate ( cis- 6c), 76:24 mixture of trans- and cis-isomers. Yield 0.39 g (79%), mp 213–214°C (decomp.). IR spectrum, ν, cm−1: 1727, 1615, 1563, 1500, 1462, 1428, 1365, 1328. 1H NMR spectrum, δ, ppm (J, Hz): trans-isomer: 2.33 (1H, t, J = 8.2, 1-CHH); 2.36 (3H, s, NCH3); 3.11 (1H, d, J = 11.9, 3-СHH); 3.69 (1H, t, J = 8.6, 1-СHH); 3.73 (3H, s, CH3O); 4.08 (1H, t, J = 7.3, 9b-CH); 4.29 (1H, d, J = 11.9, 3-СHH); 6.79 (1H, d, J = 2.8, H-9); 6.90 (1H, dd, J = 8.9, J = 2.8, H-7); 7.19 (1H, d, J = 8.9, H-6); 7.37−7.57 (5H, m, H Ph); cis-isomer: 2.12 (3H, s, NCH3); 2.30 (1H, d, J = 11.5, 3-СHH); 3.76 (3H, s, CH3O); 3.65 (1H, t, J = 8.8, 1-CHH); 4.68 (1H, t, J = 7.0, 9b-CH); 6.86 (1H, dd, J = 8.9, J = 2.8, H-7); 6.95 (1H, d, J = 2.8, H-9); 7.09 (1H, d, J = 8.9, H-6); 7.52−7.67 (5H, m, H Ph); the signals of 1-СHH and 3-СHH protons of the cis-isomer overlapped with the signals of trans-isomer. 13C NMR spectrum, δ, ppm (J, Hz): trans-isomer: 41.0 (NCH3); 43.6 (C-9b); 55.4 (CH3O); 60.3 (C-1); 60.4 (q, J = 2.4, C-3); 79.8 (q, J = 27.6, C-4); 98.2; 112.0; 114.6; 118.0; 123.2 (q, J = 289.6, CF3); 123.9; 127.1 (2C Ph); 128.4 (2C Ph); 130.0; 131.1; 142.4; 155.3; 161.1 (2C CO2H); cis-isomer: 40.7 (NCH3); 42.0 (C-9b); 55.5 (CH3O); 61.7; 62.9; 78.2 (q, J = 28.4, C-4); 97.6; 112.1; 114.3; 117.6; 123.5 (q, J = 292.7, CF3); 123.8; 126.8 (q, J = 1.6, 2C Ph); 128.4 (2C Ph); 130.1; 132.1; 142.3; 145.4; 161.1 (2C CO2H). 19F NMR spectrum, δ, ppm: trans-isomer: 95.1 (s, CF3); cis-isomer: 95.3 (s, CF3). Found, %: С 52.92; Н 4.29; N 5.73. C20H19F3N2O4·(CO2H)2. Calculated, %: С 53.02; Н 4.25; N 5.62.
(3a S *,4 R *,9b R *)-6-Ethoxy-2-methyl-3a-nitro-4-phenyl4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4- c ]pyrrole oxalate (trans-6d) and (3aS*,4S*,9bR*)-6-ethoxy2-methyl-3a-nitro-4-phenyl-4-(trifluoromethyl)-1,2,3,3a,4,9bhexahydrochromeno[3,4-c]pyrrole oxalate (cis-6d), 81:19 mixture of trans- and cis-isomers. Yield 0.39 g (76%), mp 206–207°C (decomp.). IR spectrum, ν, cm−1: 1726, 1614, 1563, 1492, 1475, 1361, 1326. 1H NMR spectrum, δ, ppm (J, Hz): trans-isomer: 1.39 (3H, t, J = 7.0, OCH2CH3); 2.29 (1H, t, J = 7.8, 1-CHH); 2.34 (3H, s, NCH3); 3.12 (1H, d, J = 11.8, 3-СHH); 3.66 (1H, t, J = 8.6, 1-СHH); 4.12 (1H, t, J = 7.4, 9b-CH); 4.15 (1H, dq, J = 10.2, J = 7.0, OCHHCH3); 4.18 (1H, dq, J = 10.2, J = 7.0, OCHHCH3); 4.25 (1H, d, J = 11.8, 3-СHH); 6.74 (1H, dd, J = 6.8, J = 1.6, H-7); 6.96−7.05 (2H, m, H-8,9); 7.36−7.46 (5H, m, H Ph); cis-isomer: 1.31 (3H, t, J = 6.9, OCH2CH3); 2.11 (3H, s, NCH3); 2.62 (1H, d, J = 11.3, 3-СHH); 3.62 (1H, t, J = 9.2, 1-CHH); 4.11 (1H, dq, J = 10.2, J = 7.0, OCHHCH3); 4.14 (1H, dq, J = 10.2, J = 7.0, OCHHCH3); 4.67 (1H, t, J = 6.8, 9b-CH); 6.89 (1H, dd, J = 6.8, J = 1.6, H-7); 6.96−7.09 (2H, m, H-8,9); 7.53−7.58 (5H, m, H Ph); the signals of 1-СHH and 3-СHH protons of the cis-isomer overlapped with the signals of the trans-isomer. 13C NMR spectrum, δ, ppm (J, Hz): trans-isomer: 14.6 (CH3); 40.8 (NCH3); 43.1 (C-9b); 60.6 (C-1); 60.9 (q, J = 2.2, C-3); 64.2 (OCH2); 79.9 (q, J = 28.1, C-4); 98.2; 112.2; 118.8; 123.2 (q, J = 289.5, CF3); 123.7; 124.8; 126.8 (2C Ph); 128.4 (2C Ph); 129.9; 131.2; 138.1; 147.5; 161.1 (2C CO2H); cis-isomer: 14.6 (CH3); 40.5 (NCH3); 41.8 (C-9b); 62.0; 63.2; 64.3 (OCH2); 78.2 (q, J = 28.2, C-4); 97.8; 112.6; 118.9; 124.6 (q, J = 292.8, CF3); 123.6; 124.8; 126.7 (q, J = 1.6, 2C Ph); 128.3 (2C Ph); 130.0; 132.3; 138.1; 147.3; 161.1 (2C CO2H). 19F NMR spectrum, δ, ppm: trans-isomer: 94.5 (s, CF3); cis-isomer: 95.1 (s, CF3). Found, %: С 54.28; Н 4.26; N 5.47. C21H21F3N2O4·(CO2H)2. Calculated, %: С 53.91; Н 4.52; N 5.47.
(3a S *,4 R *,9b R *)-6,8-Dichloro-2-methyl-3a-nitro-4-phenyl4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4- c ]pyrrole oxalate ( trans- 6e) and (3a S *,4 S *,9b R *)-6,8-dichloro-2-methyl-3a-nitro-4-phenyl-4-(trifluoromethyl)1,2,3,3a,4,9b-hexahydrochromeno[3,4- c ]pyrrole oxalate ( cis- 6e), 96:4 mixture of trans- and cis-isomers. Yield 0.30 g (68%), mp 231–232°C (decomp.). IR spectrum, ν, cm−1: 1730, 1649, 1569, 1492, 1464, 1416, 1369, 1330. 1H NMR spectrum, δ, ppm (J, Hz): trans-isomer: 2.32 (1H, t, J = 8.3, 1-CHH); 2.38 (3H, s, NCH3); 3.15 (1H, d, J = 12.1, 3-СHH); 3.71 (1H, t, J = 8.4, 1-СHH); 4.23 (1H, t, J = 7.7, 9b-CH); 4.35 (1H, d, J = 12.1, 3-СHH); 7.40 (1H, d, J = 2.1, H-7(9)); 7.45−7.57 (5H, m, H Ph); 7.71 (1H, d, J = 2.1, H-9(7)); cis-isomer: 2.14 (3H, s, NCH3); 2.31 (1H, dd, J = 9.4, J = 5.8, 1-СHH); 2.72 (1H, d, J = 11.6, 3-СHH); 3.17 (1H, d, J = 11.6, 3-СHH); 3.64 (1H, t, J = 8.8, 1-CHH); 4.79 (1H, t, J = 6.9, 9b-CH); 7.56 (1H, d, J = 2.5, H-6); 7.57−7.70 (7H, m, H-7,9, H Ph). 13C NMR spectrum, δ, ppm (J, Hz): trans-isomer: 40.7 (NCH3); 43.9 (C-9b); 60.0 (C-1); 60.2 (q, J = 2.1, C-3); 80.6 (q, J = 28.0, C-4); 97.8; 122.6; 122.9 (q, J = 289.5, CF3); 126.4; 126.6 (3C); 127.7; 128.4; 128.7 (2C Ph); 130.4 (2C); 143.7; 161.0 (2C CO2H). 19F NMR spectrum, δ, ppm: trans-isomer: 95.4 (s, CF3); cis-isomer: 94.7 (s, CF3). Found, %: С 46.83; Н 3.13; N 5.28. C19H15Cl2F3N2O3·(CO2H)2. Calculated, %: С 46.95; Н 3.19; N 5.21.
(3a S *,4 R *,9b R *)-6,8-Dibromo-2-methyl-3a-nitro-4-phenyl4-(trifluoromethyl)-1,2,3,3a,4,9b-hexahydrochromeno[3,4- c ]pyrrole oxalate ( trans- 6f). Yield 0.42 g (67%), mp 231–232°C (decomp.). IR spectrum, ν, cm−1: 1720, 1652, 1567, 1497, 1455, 1367, 1327. 1H NMR spectrum, δ, ppm (J, Hz): 2.31 (1H, t, J = 8.2, 1-CHH); 2.38 (3H, s, NCH3); 3.13 (1H, d, J = 12.1, 3-СHH); 3.71 (1H, t, J = 8.5, 1-СHH); 4.23 (1H, t, J = 7.9, 9b-CH); 4.35 (1H, d, J = 12.1, 3-СHH); 7.44−7.60 (6H, m, H-7(9), H Ph); 7.91 (1H, d, J = 1.6, H-9(7)). 13C NMR spectrum, δ, ppm (J, Hz): 40.7 (NCH3); 43.9 (C-9b); 60.0 (C-1); 60.1 (q, J = 2.1, C-3); 80.8 (q, J = 28.3, C-4); 97.7; 112.0; 115.7; 122.8 (q, J = 290.1, CF3); 126.7 (3C); 128.7 (2C Ph); 129.8; 130.3; 130.4; 133.8; 145.2; 161.0 (2C CO2H). 19F NMR spectrum, δ, ppm (J, Hz): 95.6 (s, CF3). Found, %: С 40.24; Н 2.51; N 4.54. C19H15Br2F3N2O3·(CO2H)2. Calculated, %: С 40.28; Н 2.74; N 4.57.
Purification of trans-isomers of chromeno[3,4-c]pyrrolidines 5a–f from cis-isomers. The appropriate mixture containing diastereomeric chromeno[3,4-c]pyrrolidine oxalates 6a–f (1.0 mmol) was washed with Me2CO (5×1 ml), dissolved in CH2Cl2 (3 ml), and treated with NaOH (0.05 g, 1.3 mmol) as solution in H2О (3 ml). The obtained mixture was stirred for 1 h at room temperature, the organic layer was separated and dried over anhydrous Na2SO4. The solvent was removed at reduced pressure and the solid residue was recrystallized from hexane. Chromeno[3,4-c]pyrrolidines trans-5a−f were isolated as white powders. The yields and melting points of individual trans-isomers 5a−f are presented in Table 3.
X-ray structural analysis of compound trans-5d was performed at 22°C on an Xcalibur 3 diffractometer with CCD detector according to the standard procedure (CuKα radiation, graphite monochromator, ω-scanning). Crystals suitable for X-ray structural analysis were obtained by slow evaporation of a solution of compound trans-5d in CH2Cl2. The structure of compound trans-5d was solved by direct method using the SHELX97 software suite.8 The positions of all non-hydrogen atoms were independently refined in anisotropic approximation, the hydrogen atom positions were calculated geometrically and refined according to the “rider” method with dependent temperature parameters. The complete X-ray diffraction dataset was deposited at the Cambridge Crystallographic Data Center (deposit CCDC 1862682).
References
Ievlev, M. Yu.; Ershov, O. V. Chem. Heterocycl. Compd. 2018, 54, 590. [Khim. Geterotsikl. Soedin. 2018, 54, 590.]
(a) Hancock, A. A.; Buckner, S. A.; Brune, M. E.; Esbenshade, T. A.; Ireland, L. M.; Katwala, S.; Milicic, I.; Meyer, M. D.; Kerwin, J. F., Jr.; Williams, M. J. Pharmacol. Exp. Ther. 2002, 300, 478. (b) Haight, A. R.; Bailey, A. E.; Baker, W. S.; Cain, M. H.; Copp, R. R.; DeMattei, J. A.; Ford, K. L.; Henry, R. F.; Hsu, M. C.; Keyes, R. F.; King, S. A.; McLaughlin, M. A.; Melcher, L. M.; Nadler, W. R.; Oliver, P. A.; Parekh, S. I.; Patel, H. H.; Seif, L. S.; Staeger, M. A.; Wayne, G. S.; Wittenberger, S. J.; Zhang, W. Org. Process Res. Dev. 2004, 8, 897. (c) Dubuffet, T.; Newman-Tancredi, A.; Cussac, D.; Audinot, V.; Loutz, A.; Millan, M. J.; Lavielle, G. Bioorg. Med. Chem. Lett. 1999, 9, 2059. (d) Millan, M. J.; Buccafusco, J. J.; Loiseau, F.; Watson, D. J.; Decamp, E.; Fone, K. C.; Thomasson-Perret, N.; Hill, M.; Mocaer, E.; Schneider, J. S. Int. J. Neuropsychopharmacol. 2010, 13, 1035.
(a) Meyer, M. D.; Altenbach, R. J.; Basha, F. Z.; Carroll, W. A.; Drizin, I.; Kerwin, J. F.; Wendt, M. D. US Patent 5891882. (b) Wang, C.; Yang, X.; Raabe, G.; Enders, D. Adv. Synth. Catal. 2012, 354, 2629. (c) Sato, T.; Miyazaki, T.; Arai, T. J. Org. Chem. 2015, 80, 10346. (d) Buev, E. M.; Moshkin, V. S.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2017, 53, 167. [Khim. Geterotsikl. Soedin. 2017, 53, 167.]
(a) Döndas, H. A.; Retamosa, M. G.; Sansano, J. M. Synthesis 2017, 2819. (b) Singh, M. S.; Chowdhury, S.; Koley, S. Tetrahedron 2016, 72, 1603. (c) Korotaev, V. Yu.; Sosnovskikh, V. Ya.; Barkov, A. Yu. Russ. Chem. Rev. 2013, 82, 1081. [Usp. Khim. 2013, 82, 1081.] (d) Rao, J. N. S.; Raghunathan, R. Tetrahedron Lett. 2013, 54, 6568.
(a) Nyerges, M.; Virányi, A.; Marth, G.; Dancsó, A.; Blaskó, G.; Tőke, L. Synlett 2004, 2761. (b) Korotaev, V. Yu.; Barkov, A. Yu.; Moshkin, V. S.; Matochkina, E. G.; Kodess, M. I.; Sosnovskikh, V. Ya. Tetrahedron 2013, 69, 8602.
(a) Barkov, A. Yu.; Korotaev, V. Yu.; Kotovich, I. V.; Zimnitskiy, N. S.; Kutyashev, I. B.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2016, 52, 814. [Khim. Geterotsikl. Soedin. 2016, 52, 814.] (b) Korotaev, V. Yu.; Kutyashev, I. B.; Barkov, A. Yu.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2017, 53, 1192. [Khim. Geterotsikl. Soedin. 2017, 53, 1192.]
(a) Korotaev, V. Yu.; Sosnovskikh, V. Ya.; Barabanov, M. A.; Yasnova, E. S.; Ezhikova, M. A.; Kodess, M. I.; Slepukhin, P. A. Tetrahedron 2010, 66, 1404. (b) Korotaev, V. Yu.; Sosnovskikh, V. Ya.; Barkov, A. Yu.; Slepukhin, P. A.; Ezhikova, M. A.; Kodess, M. I.; Shklyaev, Yu. V. Tetrahedron 2011, 67, 8685. (c) Korotaev, V. Yu.; Barkov, A. Yu.; Sosnovskikh, V. Ya. Tetrahedron 2013, 69, 9642. (d) Korotaev, V. Yu.; Barkov, A. Yu.; Kutyashev, I. B.; Kotovich, I. V.; Ezhikova, M. A.; Kodess, M. I.; Sosnovskikh, V. Ya. Tetrahedron 2015, 71, 2658. (e) Korotaev, V. Yu.; Kotovich, I. V.; Barkov, A. Yu.; Kutyashev, I. B.; Kodess, M. I.; Sosnovskikh, V. Ya. Tetrahedron 2016, 72, 216. (f) Korotaev, V. Yu.; Kutyashev, I. B.; Barkov, A. Yu.; Sosnovskikh, V. Ya. Tetrahedron 2017, 73, 5122. (g) Korotaev, V. Yu.; Kutyashev, I. B.; Barkov, A. Yu.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2017, 53, 597. [Khim. Geterotsikl. Soedin. 2017, 53, 597.]
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2018, 54(9), 852–858
Rights and permissions
About this article
Cite this article
Korotaev, V.Y., Kutyashev, I.B., Barkov, A.Y. et al. 3-Nitro-2-phenyl-2-(trifluoromethyl)-2H-chromenes in reaction with N-methylazomethine ylide: stereoselective synthesis of 3a,4,4-trisubstituted chromeno[3,4-c]pyrrolidines. Chem Heterocycl Comp 54, 852–858 (2018). https://doi.org/10.1007/s10593-018-2361-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-018-2361-4