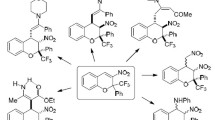
Aromatic amines reacted with 2-(trifluoroacetyl)chromones as С- or N-nucleophiles, depending on the conditions. When the reaction was performed under solvent-free conditions at 100°С for 12–18 h, they acted as С-nucleophiles and gave bishetarylcarbinols in 21–67% yields, while in refluxing toluene the addition of primary arylamines occurred via the amino group, providing the corresponding hemiaminals (80–86%).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Despite the rarity of fluorine-containing organic molecules in living nature, the trifluoromethyl group is one of the most valuable functional groups in synthetic and medicinal chemistry. The importance of this group is associated with the fact that many trifluoromethylated compounds show a variety of useful properties and find many applications in medicine and agriculture.1 Their synthesis is often based on the use of CF3-containing building blocks with relatively low molecular mass, which also must be sufficiently reactive and readily available.2, 3
We have recently described a synthesis of 2-(trifluoroacetyl)chromones, which clearly meet these requirements and easily react with 1,2-diamines, forming partially fluorinated 5,6-dihydropyrazines and quinoxalines.4 Besides that, indole and its methyl derivatives can add at the trifluoroacetyl moiety of these chromones with the formation of bishetarylcarbinols.5 Similar reactions have been interpreted in the literature as electrophilic oxyalkylation of indoles and other 5-membered hetero-cycles.6
Due to the fact that the synthetic potential of 2-(trifluoroacetyl)chromones has not yet been thoroughly explored, our goal in the current work was to study for the first time their interactions with aromatic amines. On the basis of results reported by authors from Ukraine, who demonstrated that primary, secondary, and tertiary aromatic amines reacted with 2-(trifluoroacetyl)-1,3-benzothiazole according to the scheme of ortho/para oxyalkylation,7 it could have been expected that anilines also should behave as С-nucleophiles in reactions with 2-(trifluoroacetyl)chromones.
Indeed, we found that 2-(trifluoroacetyl)chromones, which existed as covalent hydrates 1a,b (R = H, Me) due to the strongly electrophilic nature of the CF3CO group,4 reacted with aniline, N-methyl- and N,N-dimethylanilines (3.5 equiv) in the absence of solvent at 100–105°С over 12–18 h and gave bishetarylcarbinols 2a–d in 21–67% yields. Thus, the reaction proceeded as electrophilic para oxyalkylation of anilines, which served the role of С-nucleophiles in this case (Scheme 1).

Scheme 1.
The yields of aniline adducts 2а,b were merely 21–25% and could not be improved by increasing the reaction duration to 40 h. Compared to the reaction with aniline, the yields of reactions with N-methyl- and N,N-dimethylanilines were substantially higher (55–67%), which apparently can be explained by the more pronounced electron-donating character of the benzene ring. When attempting to extend the series of carbinols 2 by using 6-chloro- and 6-bromo-2-(trifluoroacetyl)chromones in the reaction with aromatic amines upon heating for 20 h, mainly the starting chromones were isolated, along with 2 to 6% of the target products (estimated from the data of 19F NMR spectroscopy).
It is interesting to note that when the reaction conditions were changed and chromone 1a was refluxed in toluene with aniline or p-anisidine (1.5 equiv) for 12 h, hemiaminals 3a,b were formed in 80 and 86% yields, respectively, via the addition of aniline amino group at the trifluoroacetyl substituent (Scheme 1). These compounds were reasonably stable during storage, but partially decomposed to the starting materials when maintained in DMSO solution. It should be noted that N-methyl- and N,N-dimethylanilines under these conditions practically did not react with chromones 1а, which was isolated in unchanged form together with 5–6% of carbinols 2c,d (estimated from the data of 19F NMR spectroscopy). At the same time, it has been reported that aromatic amines of various structures behaved as C-nucleophiles upon heating in toluene with 2-(trifluoroacetyl)-1,3-benzothiazole and reacted only at the benzene ring.7 We propose that the decreased reactivity of chromones 1 compared to 2-(trifluoroacetyl)-1,3-benzothiazole can be caused by their existence in hydrate form.
The structure of the obtained compounds was established on the basis of high-resolution mass spectrometry, IR spectroscopy, as well as 1Н, 13С, and 19F NMR spectroscopy. Comparing 1Н NMR spectra of isomers 2а and 3a showed, as should have been expected, that the otherwise similar chemical shifts of chromone ring protons were significantly altered by the presence of aniline ring. The difference in chemical shifts of the ОН group protons was 0.42 ppm and was explained by the appearance of an additional σ-electron acceptor (nitrogen atom) at the carbinol center of compound 3а, leading to enhancement of the intermolecular hydrogen bonds with solvent molecules. 19F NMR spectra of isomers 2а and 3a showed signals of the CF3 group at 89.4 and 83.1 ppm, respectively (Fig. 1).
Thus, aromatic amines participated in reactions with 2-(trifluoroacetyl)chromones as ambident nucleophiles and, depending on the reaction conditions, reacted at the para position of benzene ring (primary, secondary, and tertiary arylamines as С-nucleophiles under solvent-free conditions) or provided their amino group as a reactive site (primary arylamines as N-nucleophiles upon heating in toluene).
Experimental
IR spectra were recorded on a PerkinElmer Spectrum BX-II instrument by using an ATR accessory. 1Н, 19F, and 13C NMR spectra were acquired on a Bruker Avance II spectrometer (400, 376, and 100 MHz, respectively) in DMSO-d6 relative to solvent signals (2.49 and 39.5 ppm for 1Н and 13C nuclei, respectively); the internal standard for 19F NMR spectra was C6F6. Melting points were determined on an SMP30 apparatus.
The starting 2-(trifluoroacetyl)chromones 1a,b were obtained according to a published procedure.4
Synthesis of compounds 2a–d from chromones 1a,b and anilines (General method). A mixture of 2-(trifluoroacetyl)chromone hydrate 1a,b (0.4 mmol) and the appropriate aniline (1.4 mmol) was heated on a glycerol bath to 100–105°C for 12–18 h. The reaction mixture was then cooled to room temperature, diluted with 95% ethanol (2 ml), the precipitate was filtered off and washed with ethanol (1 ml), providing compounds 2a–d as yellow powders.
2-[1-(4-Aminophenyl)-1-hydroxy-2,2,2-trifluoroethyl]-4 H -chromen-4-one (2а). Yield 49 mg (25%), mp 229–230°C. IR spectrum, ν, cm–1: 3288, 1636, 1617, 1584, 1568, 1482. 1H NMR spectrum, δ, ppm (J, Hz): 5.36 (2Н, br. s, NH2); 6.57 (2Н, d, J = 8.6, Н-3',5'); 6.60 (1Н, s, 3-CH); 7.24 (2Н, d, J = 8.6, Н-2',6'); 7.53 (1Н, td, J = 7.6, J = 0.8, Н-6); 7.61 (1Н, d, J = 8.5, Н-8); 7.74 (1Н, s, ОН); 7.82 (1Н, ddd, J = 8.5, J = 7.2, J = 1.6, Н-7); 8.06 (1Н, dd, J = 7.9, J = 1.6, Н-5). 13C NMR spectrum, δ, ppm (J, Hz): 77.1 (q, 2JCF = 28.7); 109.7; 113.3; 118.4; 121.8 (d, 3JCF = 1.8); 123.0; 124.4 (q, 1JCF = 287.6); 124.9; 126.0; 127.5; 134.8; 149.5; 155.4; 165.6; 176.7. 19F NMR spectrum, δ, ppm: 89.4 (s, CF3). Found, m/z: 336.0839 [M+H]+. С17Н13F3NO3. Calculated, m/z: 336.0832.
2-[1-(4-Aminophenyl)-1-hydroxy-2,2,2-trifluoroethyl]-6-methyl-4 H -chromen-4-one (2b). Yield 36 mg (21%), mp 235–236°C. IR spectrum, ν, cm–1: 3433, 3377, 3260, 3191, 3074, 2776, 2668, 1643, 1615, 1576, 1515, 1500, 1483. 1H NMR spectrum, δ, ppm (J, Hz): 2.43 (3Н, s, CH3); 5.36 (2Н, br. s, NH2); 6.56 (2Н, d, J = 8.6, Н-3',5'); 6.57 (1Н, s, 3-CH); 7.22 (2Н, d, J = 8.6, Н-2',6'); 7.51 (1Н, d, J = 8.6, Н-8); 7.64 (1Н, dd, J = 8.6, J = 2.2, Н-7); 7.72 (1Н, s, ОН); 7.84 (1Н, d, J = 2.0, Н-5). 13C NMR spectrum, δ, ppm (J, Hz): 20.4; 77.1 (q, 2JCF = 28.6); 109.6; 113.2; 118.2; 121.8; 122.8; 124.1; 124.5 (q, 1JCF = 287.7); 127.5; 128.7; 135.8; 149.5; 153.7; 165.5; 176.7. 19F NMR spectrum, δ, ppm: 89.3 (s, CF3). Found, m/z: 350.0988 [M+H]+. С18Н15F3NO3. Calculated, m/z: 350.0999.
2-[1-Hydroxy-1-(4-methylaminophenyl)-2,2,2-trifluoroethyl]-4 H -chromen-4-one (2c). Yield 70 mg (55%), mp 176–177°C. IR spectrum, ν, cm–1: 3241, 3111, 2932, 2905, 2823, 1634, 1574, 1532, 1480. 1H NMR spectrum, δ, ppm (J, Hz): 2.66 (3Н, d, J = 4.7, CH3); 5.94 (1Н, q, J = 4.7, NH); 6.55 (2Н, d, J = 8.7, Н-3',5'); 6.60 (1Н, s, 3-CH); 7.31 (2Н, d, J = 8.7, Н-2',6'); 7.53 (1Н, t, J = 7.5, Н-6); 7.60 (1Н, d, J = 8.4, Н-8); 7.77 (1Н, s, ОН); 7.82 (1Н, ddd, J = 8.6, J = 7.2, J = 1.6, Н-7); 8.06 (1Н, dd, J = 7.9, J = 1.6, Н-5). 13C NMR spectrum, δ, ppm (J, Hz): 29.4; 77.1 (q, 2JCF = 28.6); 109.7; 111.1; 118.4; 121.5; 123.0; 124.9; 126.0; 127.3 (q, 1JCF = 287.5); 127.5; 134.8; 150.4; 155.4; 165.6; 176.7. 19F NMR spectrum, δ, ppm: 89.3 (s, CF3). Found, m/z: 350.1007 [M+H]+. С18Н15F3NO3. Calculated, m/z: 350.0999.
2-[1-Hydroxy-1-(4-dimethylaminophenyl)-2,2,2-trifluoroethyl]-4 H -chromen-4-one (2d). Yield 142 mg (67%), mp 198–199°C. IR spectrum, ν, cm–1: 3272, 2905, 2819, 1641, 1614, 1569, 1531, 1464. 1H NMR spectrum, δ, ppm (J, Hz): 2.90 (6Н, s, 2CH3); 6.61 (1Н, s, 3-CH); 6.74 (2Н, d, J = 9.0, Н-3',5'); 7.40 (2Н, d, J = 9.0, Н-2',6'); 7.53 (1Н, t, J = 7.6, Н-6); 7.61 (1Н, d, J = 8.5, Н-8); 7.82 (1Н, ddd, J = 8.5, J = 7.3, J = 1.6, Н-7); 7.84 (1Н, s, ОН); 8.06 (1Н, dd, J = 7.9, J = 1.5, Н-5). 13C NMR spectrum, δ, ppm (J, Hz): 39.7; 77.1 (q, 2JCF = 28.8); 109.7; 111.6; 118.3; 122.0; 123.0; 124.4 (q, 1JCF = 288.1); 124.9; 126.0; 127.4; 134.7; 150.6; 155.4; 165.4; 176.6. 19F NMR spectrum, δ, ppm: 89.3 (s, CF3). Found, m/z: 364.1161 [M+H]+. C19H16F3NO3. Calculated, m/z: 364.1155.
2-(1-Anilino-1-hydroxy-2,2,2-trifluoroethyl)-4 H -chromen-4-one (3a). A solution of 2-(trifluoroacetyl)chromone (1а) (150 mg, 0.58 mmol) and aniline (80 mg, 0.86 mmol) in toluene (5 ml) was refluxed for 12 h and left overnight. The precipitate that formed was filtered off and washed with toluene (1 ml). Yield 155 mg (80%), white powder, mp 137–138°C. IR spectrum, ν, cm–1: 3339, 3201, 3120, 3060, 1638, 1598, 1571, 1531, 1501, 1483. 1H NMR spectrum, δ, ppm (J, Hz): 6.67 (1Н, t, J = 7.3, H-4'); 6.72 (1Н, s, 3-CH); 6.99 (2Н, d, J = 7.7, Н-2',6'); 7.07 (2Н, t, J = 7.9, Н-3',5'); 7.20 (1Н, s, NH); 7.50 (1Н, t, J = 7.6, Н-6); 7.55 (1Н, d, J = 8.5, Н-8); 7.80 (1Н, ddd, J = 8.5, J = 7.2, J = 1.5, Н-7); 8.02 (1Н, dd, J = 7.9, J = 1.5, Н-5); 8.16 (1Н, s, ОН). 13C NMR spectrum, δ, ppm (J, Hz): 83.2 (q, 2JCF = 30.6); 112.3; 113.8; 116.2; 118.3; 119.0; 122.9 (q, 1JCF = 290.6); 125.9; 126.0; 128.5; 134.8; 143.8; 148.5; 162.3; 176.5. 19F NMR spectrum, δ, ppm: 83.1 (s, CF3). Found, m/z: 336.0844 [M+H]+. С17Н13F3NO3. Calculated, m/z: 336.0842.
2-{1-Hydroxy-1-[(4-methoxyphenyl)amino]-2,2,2-trifluoroethyl}-4 H -chromen-4-one (3b) was obtained from chromone 1а and anisidine analogously to the procedure for preparation of compound 3а. Yield 123 mg (86%), yellow powder, mp 134–135°C. IR spectrum, ν, cm–1: 3356, 3187, 2838, 1637, 1599, 1569, 1515, 1483, 1467. 1H NMR spectrum, δ, ppm (J, Hz): 3.59 (3Н, s, CH3); 6.66 (1Н, s, 3-CH); 6.68 (2Н, d, J = 9.0, Н-3',5'); 6.74 (1Н, br. s, NH); 6.96 (2Н, d, J = 9.0, Н-2',6'); 7.50 (1Н, ddd, J = 7.9, J = 7.2, J = 0.7, Н-6); 7.57 (1Н, d, J = 8.4, Н-8); 7.81 (1Н, ddd, J = 8.4, J = 7.2, J = 1.6, Н-7); 8.01 (1Н, dd, J = 7.9, J = 1.6, Н-5); 8.06 (1Н, s, ОН). 13C NMR spectrum, δ, ppm (J, Hz): 54.9; 83.8 (q, 2JCF = 30.4); 112.2; 113.9; 114.9; 118.4; 124.1 (q, 1JCF = 294.4); 124.9; 126.0; 128.2; 128.9; 136.4; 153.1; 155.6; 162.6; 176.6. 19F NMR spectrum, δ, ppm: 83.4 (s, CF3). Found, m/z: 366.0953 [M+H]+. C18H15F3NO4. Calculated, m/z: 366.0948.
References
(a) Organofluorine Compounds in Medicinal Chemistry and Biomedical Applications; Filler, R.; Kobayashi, Y.; Yagupolskii, L. M., Eds.; Elsevier: Amsterdam, 1993. (b) Hiyama, T. Organofluorine Compounds: Chemistry and Application; Springer: Berlin, 2000. (c) Kirk, K. L. J. Fluorine Chem. 2006, 127, 1013. (d) Bégué, J.-P.; Bonnet-Delpon, D. J. Fluorine Chem. 2006, 127, 992. (e) Dolbier, W. R., Jr. J. Fluorine Chem. 2005, 126, 157.
(a) Lin, P.; Jiang, J. Tetrahedron 2000, 56, 3635. (b) Druzhinin, S. V.; Balenkova, E. S.; Nenajdenko, V. G. Tetrahedron 2007, 63, 7753. (c) Nenajdenko, V. G.; Balenkova, E. S. ARKIVOC 2011, (i), 246. (d) Sosnovskikh, V. Y. Russ. Chem. Rev. 2003, 72, 489. [Usp. Khim. 2003, 72, 550.] (e) Isakova, V. G.; Khlebnikova, T. S.; Lakhvich, F. A. Russ. Chem. Rev. 2010, 79, 849. [Usp. Khim. 2010, 79, 929.] (f) Korotaev, V. Y.; Sosnovskikh, V. Y.; Barkov, A. Y. Russ. Chem. Rev. 2013, 82, 1081. [Usp. Khim. 2013, 82, 1081.]
Sosnovskikh, V. Y. In Fluorine in Heterocyclic Chemistry; Nenajdenko, V., Ed.; Springer: Cham, 2014, Vol. 2, p. 211.
Irgashev, R. A.; Safrygin, A. V.; Ezhikova, M. A.; Kodess, M. I.; Röschenthaler, G.-V.; Sosnovskikh, V. Y. Tetrahedron 2015, 71, 1822.
Safrygin, A. V.; Irgashev, R. A.; Barabanov, M. A.; Sosnovskikh, V. Y. Tetrahedron 2016, 72, 227.
(a) Khodakovskiy, P. V.; Mykhailiuk, P. K.; Volochnyuk, D. M.; Tolmachev, A. A. Synthesis 2010, 967. (b) Khodakovskiy, P. V.; Mykhailiuk, P. K.; Volochnyuk, D. M.; Tolmachev, A. A. Synthesis 2010, 979. (c) Khodakovskiy, P. V.; Mykhailiuk, P. K.; Volochnyuk, D. M.; Tolmachev, A. A. Synthesis 2010, 1195.
Khodakovskiy, P. V.; Mykhailiuk, P. K.; Volochnyuk, D. M.; Tolmachev, A. A. Synthesis 2010, 1633.
This work received financial support from the Russian Foundation for Basic Research (grant No. 17-03-00340).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2017, 53(12), 1362–1364
Rights and permissions
About this article
Cite this article
Safrygin, A.V., Vetyugova, D.A., Irgashev, R.A. et al. Reactions of 2-(trifluoroacetyl)chromones with aromatic amines. Chem Heterocycl Comp 53, 1362–1364 (2017). https://doi.org/10.1007/s10593-018-2218-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-018-2218-x