The diverse biological activities of compounds containing N-(hetero)aryl-2-imidazoline moiety and methods to construct them are comprehensively reviewed for the first time. This intriguing non-flat, cyclic amidine motif clearly represents an emerging privileged structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
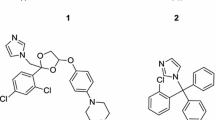
2-Imidazolines represent a distinctly important case of cyclic N,N'-dialkylamidines, as they act as primary modulators of an entire family of cell surface receptors which are expressed in the central nervous system and, notably, were designated imidazoline receptors due to the high affinity to 4,5-dihydro-1H-imidazole-containing compounds.1 2-Imidazoline scaffold is central to numerous biologically active compounds whose targets are vastly different from imidazoline receptors, as was reviewed recently.2 Prominent examples include estrogen receptor modulator 1 for oncology applications,3 proteasome inhibitor 2 for multiple myeloma,4 P2X7 ion channel blocker 3 useful in the treatment of inflammatory conditions,5 and Nutlin-3 (4)6 known to disrupt the oncogenic p53-mdm2 protein–protein interactions (Fig. 1). Thus, one can confidently regard the 2-imidazoline core as a privileged scaffold for drug design, as defined by Evans et al.7 Acyclic N-(hetero)aryl amidines have been richly exemplified in the synthetic chemistry literature8 , 9 and can be accessed via a number of arylation protocols from the parent amidine substrates. N-Arylation of substrates containing a nucleophilic (basic) nitrogen atom is an important modification in the practice of medicinal chemistry the consequences of which are generally twofold. On the one hand, introduction of an aryl (or heteroaryl) substituent at a nitrogen atom attenuates its basicity. On the other, addition of an aromatic group increases compound's lipophilicity and creates new opportunities of building specific contacts with a protein target via hydrophobic and π-stacking interactions.10 Hence, various acyclic N-(hetero)arylamidines have found utility in the medicinal chemistry design.11 The same, however, could not be said about N-(hetero)aryl-2-imidazolines even five years ago when only a handful of examples had been reported in the literature.
In 2012, we became puzzled by the absence of dedicated methodology studies on the N-(hetero)arylation of 2-imidazolines and developed12 a Buchwald–Hartwig-type method to prepare lead-like13 and even fragment-like14 N-(hetero)aryl-2-imidazolines as discussed below. Capitalizing on this methodology, we have now identified a number of bioactive chemical series that employ the said N-(hetero)arylation as a primary scaffold-generating event. Considering the range of diverse biological activities identified for these compounds based on this scaffold in relatively short period of time, we would like to designate N-(hetero)aryl-2-imidazolines as an emerging privileged motif useful for the drug design and aim to summarize the chemistries and the biological data substantiating this motion in present review.
Early (pre-2012) examples of 2-imidazoline N -(hetero)arylation
Until dedicated methodological efforts on metalcatalyzed N-(hetero)arylation of 2-imidazolines commenced in 2012,12 the literature reports amounted to a handful of isolated examples, primarily in the patent literature, which included either direct S NAr-type N-(hetero)-arylation or a few cases of the same reaction brought about under Pd- or Cu-catalyzed conditions.
Examples of direct S NAr using 2-imidazoline as a nucleophile are limited to very active aromatic electrophilic partner. The first example ever encountered was a part of a 1975 study, reported by Servier researchers, directed toward development of antibacterials. In this work, a series of 2-imidazoline-substituted 6,8-diazaquinolonic acid esters 6 was produced by reacting their sulfone precursor (generated in situ from compound 5) with appropriate 2-imidazolines (Scheme 1).15

Scheme. 1
The interest to N-(hetero)aryl-2-imidazoline moieties for medicinal chemistry design seemed to have been dormant for over 25 years, i.e., until 2002 when Aventis scientists used 2-methylimidazoline to displace chlorine in precursor 7 en route to telomerase inhibitor 8 via a base-promoted S NAr reaction (Scheme 2, yield not reported).16

Scheme. 2
A similar reaction with 4-chloropyrimidine 9 reported by Actelion Pharmaceuticals required much less forcing conditions and delivered P2Y12 receptor antagonist 10, representative of a useful class of antithrombotic agents (Scheme 3, yield not reported).17

Scheme. 3
N-Heteroarylation of 2-methylimidazoline with 2-chloropyrimidine 11 reported in the preparation of p38 MAP kinase inhibitor 12 by Glaxo required that the methylsulfanyl group be oxidized to a more labile methyl sulfone prior to the displacement (Scheme 4, yield not reported).18

Scheme. 4
The previous three examples included SNAr events at the pyrimidine or 1,3,5-triazine nucleus. Korean scientists described a similar chlorine displacement at 6-anilino-5-nitropyridine 13 to produce compound 14 (Scheme 5) investigated for inhibition of osteoblast differentiation, a promising approach for the treatment of osteoporosis.19

Scheme. 5
The only example of Cu-catalyzed N-arylation of 2-imidazolines encountered in the literature prior to 2012 is the preparation, by the Roche scientists, of JAK and SYK kinase inhibitors via intermediate 16 synthesized, in turn, via N-heteroarylation of 2-methylimidazoline with pyrrolopyrazine bromide 15 (Scheme 6, yield not reported).20

Scheme. 6
Reports on Pd-catalyzed reactions are similarly scarce prior to 2012. Only one such example, Pd2(dba)3–BINAPcatalyzed and somewhat low-yielding N-arylation of 2-(4-pyridyl)imidazoline with 5-bromoisoindolinone 17, was disclosed by AstraZeneca as a part of a large set of metabolotropic glutamate receptor potentiators exemplified by compound 18 (Scheme 7).21

Scheme. 7
Dedicated methodology studies toward 2-imidazoline N -(hetero)arylation
Lead-13 and fragment-like14 compounds are scarce in the world's modern arsenal of screening libraries. Aiming to fill that void, we paid a particular attention to N-(hetero)-aryl-2-imidazolines as a rich source of such compounds. The absence of robust, amply exemplified methodology studies toward these compounds in the literature (vide supra) prompted us to investigate and develop a Pd-catalyzed Buchwald–Hartwig-type arylation protocol (Scheme 8) that was found applicable to a range of 2-imidazolines 19 and various electron-deficient (hetero) aromatic halides (the scope of the reaction is concisely captured by selected products 20 shown in Table 1).12 The molecular metrics presented (molecular weight (MW) and partition coefficient (cLogP)22) clearly attests to these compounds' being well within the limits of lead-likeness and fragment-likeness (defined by the 'rule-of-three', i.e., MW < 300 and cLogP < 3.0).23

Scheme. 8
The Buchwald–Hartwig N-arylation protocol, despite the somewhat forcing conditions required to achieve good conversions in this reaction, was found to be compatible with a 2-imidazoline moiety already present in the heteroaryl halide partner (like in compound 21). This
finding allowed us to bring about a second Pd-catalyzed coupling without the disruption of the 2-imidazoline linkage – either with another 2-imidazoline moiety or with a secondary amine – to produce compounds 22 and 23a,b, respectively (Scheme 9).12 The possibility to perform sequential "imidazolination" of dihalo(hetero)aromatic substrates found a particular utility in the design of bioactive compounds discussed below.

Scheme. 9
The Pd-catalyzed Buchwald–Hartwig arylation displayed an obvious limitation of scope, namely, being workable only for highly reactive, electron-deficient halo(hetero)aromatics. In 2013, the Bull team from Imperial College London described a ligand-free, CuBr-catalyzed approach to N-(hetero)aryl-2-imidazolines (Scheme 10), which employed a wider range of iodo(hetero)aromatic reagents, including electron-neutral and even electron-rich ones, as illustrated in Figure 2.24

Scheme. 10
Both methods described above suffer from the same drawback, namely, requiring elevated temperatures to achieve conversion to the product. This makes the methods inapplicable to thermally-unstable substrates. To circumvent this obstacle, we developed a Chan–Evans–Lam copper(II)-promoted N-(hetero)arylation of 2-imidazolines (Scheme 11) using a collection of boronic acids. This finding allowed not only tapping into the vast reagent space of aromatic boronic acids (commercially available or ad hoc synthesized), but also conducting the (hetero)arylation reaction at ambient temperature while achieving full conversions and excellent product yields (Scheme 11, Fig. 3).25

Scheme. 11
Interestingly, the same protocol was found inapplicable to the N-arylation of 1,4,5,6-tetrahydropyrimidine 24 as it yielded only a trace amount of the anticipated product 25 (Scheme 12). This was in line with the previous report26 where Pd-catalyzed N-(hetero)arylation of 1,4,5,6-tetrahydropyrimidine was only marginally effective, which attests to 2-imidazoline scaffold's being a stand-alone class of heterocycles rather than a mere version of a cyclic amidine.

Scheme. 12
Isoform-selective inhibitors of human cyclooxygenase-2 and carbonic anhydrase
Vicinal diaryl heterocycles 26 in which one of the aromatic rings is substituted with an amino- or methylsulfonyl group represent an important class of non-steroidal anti-inflammatory agents, selective cyclooxygenase-2 (COX-2) inhibitors, useful in treatment of such debilitating chronic condition as rheumatoid arthritis. This class of drugs is represented, among others, by the blockbuster drug celecoxib (Celebrex®) (27).27 The latter drug was originally developed by G. D. Searle & Co. (now a fully-owned subsidiary of Pfizer) as part of a large-scale project exploring various heterocyclic chemotypes besides pyrazole present in compound 27. One of the advanced compounds displaying a marked COX-2 potency (and selectivity vs COX-1) was imidazole derivative 28 28 (Fig. 4).
Considering the structure 28, we reasoned that the published synthesis of this compound28 can be altered to include an N-arylation step of 2-imidazoline developed by us earlier.12 This turned out to be true, as N-arylation of 4-methylimidazoline 29 with 1-bromo-4-methylsulfonylbenzene (30) proceeded with a high yield (likely due to the electrondeficient character of sulfone 30) and regiospecifically at the less hindered nitrogen atom (ratio of isomers >10:1, identity confirmed by NOESY spectrum of product 31). The latter was aromatized into imidazoline 28 by dehydrogenation on Pd/C (Scheme 13).29

Scheme. 13
While being short and high-yielding, the above synthetic route included a cumbersome aromatization step. This gave us an idea to consider N-arylimidazolines (like compound 31) as potential COX-2 inhibitors. At the onset, it was considered a risky move due to the popular dogma articulated in the literature30 that potent and selective COX-2 inhibitors could be designed only around flat, aromatic, and relatively lipophilic cores (of which 2-imidazoline is neither). To verify this bold hypothesis, we performed a Pd-catalyzed N-arylation of a set of 15 2-imidazolines 32 (selected on the basis of the SAR information for imidazole inhibitors28) with either sulfone 30 or with 2,4-dimethoxybenzyl-protected (DMB-protected) sulfonamide 33 er (Scheme 14). This resulted in the set of 15 methylsulfone compounds 35 and 15 primary sulfonamides 36 (obtained after removal of the DMB groups in the initial N-arylation products 34).31

Scheme. 14
While methylsulfone derivatives 35 turned out completely inactive against COX-1 and COX-2, one of primary sulfonamides (compound 36n) displayed a COX-2 inhibitory potency comparable to that of celecoxib (27) and also a better COX-2 selectivity (Fig. 5).32
The presence of the primary sulfonamide moiety in compound 36 prompted us to investigate the above series of 15 compounds as inhibitors of various isoforms of human carbonic anhydrase (hCA).33 This ubiquitous enzyme superfamily is known to be inhibited by primary sulfonamides due to the latter group binding to the enzyme prosthetic zinc ion.34 As shown in Table 2, compounds 36a–o displayed an excellent (pKi 7–8) inhibitory profile against hCAII (a cytosolic anti-glaucoma and anti-edema biological target) and hCAVII (also a cytosolic target believed to be involved in epilepsy and neuropathic pain) and a marked (1–2 orders of magnitude) selectivity (SI) against cytosolic isoform hCAI and membrane-bound isoform hCAIV.
Antitubercular 2-imidazoline-based compounds
N-Heteroaryl-2-imidazoline moiety has been explored35 as a scaffold for the design of a 2-(quinolin-4-yl)-imidazoline library focused to display antimalarial and/or antitubercular activity as shown in Figure 6.
Compounds exemplifying the library design were synthesized according to the sequential Buchwald–Hartwig protocol (Scheme 15) employing 2-(quinolin-4-yl)imidazoline (37) and a range of dihaloazines 38 in combination with primary and secondary amines. The first Pd-catalyzed arylation proceeded with good yield and gave core N-(hetero)aryl-2-imidazoline building blocks 39a–e. The latter were aminated in a combinatorial fashion, and the resulting compounds 40–75 were obtained in good to excellent yields as well (Table 3).

Scheme. 15
Despite the initial expectations, none of compounds 40–75 displayed a specific antimalarial activity in a 3-day SYBR green I growth and proliferation assay.36 However, compounds 53 and 55 displayed a moderate activity against Mycobacterium tuberculosis H37Rv and no noticeable cytotoxicity, thus manifesting themselves as specific antimycobacterial agents (Fig. 7).35
Selective kinase inhibitors
N-Azine-substituted 2-imidazoline moiety was envisioned as a nonclassical binder to the so-called "hinge region" in the ATP-binding pocket of protein tyrosine kinases.37 Due to the high basicity (pK a ~4–5) such a moiety is likely to be protonated and is likely to participate in the hydrogen-bonding interaction with the hinge region of the protein backbone. The additional appendages on the azine moiety can be viewed as the basis for providing additional interactions within the ATP-binding pocket, such as additional hydrogen-bonding and hydrophobic interactions (Fig. 8).
The number of possible compounds within this chemotype is enormous, and in order to reduce it to a small number of promising candidates, we generated a structurally diverse virtual library of 1,235 compounds and screened it in silico against a panel of 48 therapeutically relevant human kinases. The virtual screening delivered five top-scoring candidate molecules 76–80 (Fig. 9).38 Compounds 76–80 were synthesized using the sequential Buchwald–Hartwig coupling approach described above (Scheme 16).39 These compounds were screened against a panel of 48 human kinases, and the data revealed that three of the five compounds tested exhibited >30% inhibition of only a few therapeutically relevant kinases: IRK (compound 76), TRKA (compound 78), Src (compound 78), CDK1/2 (compound 80), TAOK2 (compound 80). Concentration–response retesting of the same compound–kinase pairs confirmed compound 78 as a ~10 μM inhibitor of TRKA and Src, compound 80 – as a ~10 μM inhibitor of CDK1/2, TAOK2. While the inhibitory activity of compounds 78 and 80 appears to be low compared to clinically used kinase inhibitors, the two compounds represent valuable starting points for further development, in a similar fashion to the new 4–470 μM kinase inhibitory cores present in the commercial domain.40

Scheme. 16
N -(Hetero)arylation of 2-imidazolines en route to other novel and privileged scaffolds
In 2013, when attempting to reduce the nitro group in compound 81 using the so-called Bechamp conditions (Fe, NH4Cl, EtOH, H2O, heating), we observed an unexpected formation of imidazo[4,5-b]pyridine derivative 82, and not the expected N-heteroaryl-2-imidazoline 83 (Scheme 17).41

Scheme. 17
Imidazo[4,5-b]pyridines containing 2-aminoethyl side chain such as compound 82 are bioisosteres of privileged benzimidazoles42 and can be regarded as privileged structures on their own, as they have been reported as histamine H3 receptor inverse agonists,43 histone deacetylase inhibitors,44 and cannabinoid CB2 receptor modulators.45 The formation of compound 82 (instead of compound 83) can be rationalized by the hydrolytic instability46 of the 2-imidazoline linkage which can lead to expulsion of the 2-aminoethyl side chain, resulting in intermediate 84 (isolated in a control experiment performed in the absence of Fe), followed by the nitro group reduction and cyclodehydration to form compound 82 (Scheme 18).

Scheme. 18
This serendipitous discovery essentially led to a fundamentally new method (Scheme 19) to prepare a range of 2-aminoethyl-containing imidazo[4,5-b]pyridines and benzimidazoles, some examples of which are shown in Figure 10.41

Scheme. 19
Examples of imidazo[4,5-b]pyridines and benzimidazoles accessible as shown in Scheme 19 (yields given are for the first/second step).
Compound 82 can be viewed as a substrate for the second, intramolecular Buchwald–Hartwig N-arylation process. Indeed, under the Pd-catalyzed conditions it furnished rare tetracyclic compound 85 (Scheme 20) the structure of which was confirmed by X-ray crystallography.47

Scheme. 20
This novel core can be further diversified on the basis of on the following considerations. Firstly, the aminoethyl side chain can be reductively alkylated prior to the intramolecular N-arylation step, thus introducing alkyl groups at the nitrogen atom of the seven-membered (diazepine) ring. Secondly, considering the generality of the approach to 2-aminoethyl-substituted imidazo[4,5-b]pyridines and benzimidazoles presented above, the phenyl and pyridine rings can perhaps contain independently variable substituents. This was indeed realized in the general approach presented in Scheme 21.47 The range of tetracyclic compounds akin to structure 85 resulting therefrom is illustrated by the examples shown in Figure 11.

Scheme. 21
"Decorated" versions of tetracyclic compound 85 accessed as shown in Scheme 20.
An interesting opportunity to achieve 2-imidazoline N-arylation in the absence of any transition metal catalyst was identified last year when 2-(2-hydroxyphenyl)imidazolines 86, envisioned as a bis-nucleophiles, were introduced into the reaction with reactivity-matched bis-electrophilic aromatic substrates.48 The compounds expected to result therefrom were tetracyclic [1,4]oxazepines 87 which are analogs of mirtazapine, a selective noradrenaline reuptake inhibitor (Fig. 12).
The strategy was indeed realized in practice and resulted in the general method to access compounds 87 in a practically convenient and atom-economical fashion (Scheme 22).48

Scheme. 22
The fact that in order to achieve the challenging N-arylation of the 2-imidazoline moiety no metal-based catalysis was required can be rationalized in this case by the intramolecular character of this S NAr event. Indeed, as it has been shown previously49 for analogous ''2 × S N Ar'' reactions, the reaction begins by phenoxide anion displacing the most labile leaving group in the aromatic partner. A Smiles rearrangement (which in this case is essentially an intramolecular 2-imidazoline N-arylation step!) then follows, and the cyclization is completed by yet another intramolecular displacement by the phenoxide (as shown for the reaction between 2-(2-hydroxyphenyl)-imidazoline and 2,3-dichloro-5-trifluoromethylpyridine in Scheme 23).48 The range of tetracyclic products 87 that can be accessed by this remarkably simple yet efficient methodology is illustrated by the examples shown in Figure 13. Notably, here, too, the N-arylation of the methylsubstituted imidazoline takes place at the less sterically hindered nitrogen atom. However, the regiospecificity in this case appear lower compared to the isomeric ratio observed earlier for compound 31 (Scheme 13). This is unsurprising, as the selectivity can be expected to be lower between two intramolecular events which proceed with lower activation barriers in comparison to intermolecular reactions.

Scheme. 23
Examples of tetracyclic 2-imidazoline-containing products 87 accessed as shown in Scheme 23.
Recently, we have identified50 an intriguing reactivity aspect of tetracyclic 2-imidazoline-containing [1,4]oxazepines 87 which is a corollary of the hydrolytic instability of the 2-imidazoline (and especially, 2-imidazolinium) moiety, the synthetic utility of which we have already described above. Representative compound of this class (compound 88) can be regiospecifically methylated at the 2-imidazoline moiety furnishing the reactive imidazolinium salt 89. The latter reacts further in 0.1% aqueous potassium carbonate solution at room temperature. However, the result is not a 2-aminoethyl side chain-containing compound (vide supra), but rather a ring-expanded 8-methyl-2-nitro-5,6,7,8-tetrahydro-9H-benzo[i]pyrido[3,2-b][1,4,7]-oxadiazecin-9-one (90) obtained in a good overall yield (Scheme 24). The structure of compound 90 was confirmed by single-crystal X-ray analysis. In light of the general scarcity of medium-sized ring compounds in contemporary screening collections,51 compounds like compound 90 represent a rather fortunate trophy from our quest into 2-imidazoline N-arylation.

Scheme. 24
Non-arylation methods to construct N -(hetero)aryl-2-imidazolines
There is a number of reports in the literature describing the synthesis of N-(hetero)aryl-2-imidazolines by routes other than direct N-(hetero)arylation of an NH-imidazoline (mostly involving cyclodehydration of an appropriately substituted ethylenediamine precursor). Some of these studies were conducted in the context of developing 2-imidazoline derivatives endowed with specific biological activity. Therefore, we find it appropriate to present a summary of these reports in present review. We also would like to briefly review dedicated methodology studies aimed at 2-imidazoline formation where N-(hetero)aryl versions of this heterocycle have been exemplified.
An early report on the synthesis of N-aryl-2-imidazolines (termed "1-aryl-4:5-dihydroglyoxalines") was published in 1949 by the Partridge group from University of Nottingham52 (although the first preparation of N-aryl-5-methyl-imidazolines from N-allylamides and arylamine hydrochlorides or from anilides and allylamine hydrochlorides was published as far back as 1895,53 see also Scheme 29). They described a reaction between N-(2-chloroethyl)-enzamide (91) and PCl5 conducted in presence of aniline. The initially formed imidoyl chloride 92 is trapped by aniline to form amidinium salt 93. The latter was extremely prone to cyclization and afforded in an excellent yield the desired N-phenyl-2-imidazoline 94 which was isolated as a picrate salt (Scheme 25).52

Scheme. 25
N-(Imidoyl)aziridines 95 (obtained by the reaction of imidoyl chloride 96 with aziridines) were found to rearrange into respective N-aryl-2-imidazolines 97 on treatment with sodium iodide or potassium thiocyanate (Scheme 26).54 This curious entry into the heterocycle in question is vaguely reminiscent of (though mechanistically quite different from) a recently described radical rearrangement of aziridine precursor 98 into compound 99 which contains an N-aryl-2-imidazolines moiety (Scheme 27).55

Scheme. 26

Scheme. 27
The cyclodehydration of N-aryl-N'-acylethylenediamine precursor 100 into respective N-aryl-2-imidazoline 101 (Scheme 28) was described in 196656 and, later, various versions of essentially this particular powerful approach have been employed in preparation of many medicinally relevant N-aryl-2-imidazolines (vide infra).

Scheme. 28
In 1973, Partridge and Smith re-visited57 the cyclocondensation of N-allyl-N'-arylamidines (which had been alleged53 to be intermediates in the transformation of N-allyl amides and arylamine hydrochlorides or from anilides and allylamine hydrochlorides into N-aryl-5-methylimidazolines) and confirmed that isolated acetamidine hydrochlorides 102 indeed produce 1-aryl-2,5-dimethylimidazolines 103 on heating (Scheme 29).

Scheme. 29
In 2006, Orelli and coworkers employed a microwaveassisted cyclodehydration of N-acyl-N'-arylethylenediamines 104 into N-aryl-2-imidazolines 105 using ethyl polyphosphate (PPE) as dehydrating agent (Scheme 30).58 The main advantage of the microwave-promoted reaction over conventional heating was the markedly shorter reaction time (1–2 min vs 5 h). However, the yields obtained in both protocols were comparable. Curiously, in 2012, a different team from the same institution applied microwave radiation to promote cyclization of precursor 104 into imidazoline 105 using trimethylsilyl polyphosphate.59

Scheme. 30
In 2009, Khvat and coworkers described two practical synthetic routes toward N-phenyl-2-imidazolines 106 (Scheme 31) and 107 (Scheme 32) varying only in the position of the second phenyl substituent in the 2-imidazoline ring.60 Since cyclodehydration was the key step in both approaches, the preparation of the two regioisomers, in principle, only mandated judicious crafting of the cyclodehydration precursors. Interestingly, compound 107 was only one of the two products in the last step of the second sequence which involved reductive amination with ammonium acetate and spontaneous cyclodehydration of the intermediate primary aliphatic amine. The latter, however, represented an "easy target" for the N→N acyl migration that resulted in compound 108 which did not cyclize into 107 spontaneously (due to the reduced nucleo-philicity of the aniline nitrogen) and required more forcing dehydration conditions to achieve full conversion (Scheme 32).

Scheme. 31

Scheme. 32
In 2012, Jiang and coworkers synthesized a small set of N-phenyl-2-imidazolines 109 containing various fluoroalkyl substituents.61 This approach represented essentially the following sequence of events realized in a one-pot format (in which both triethylamine and the PPh3–CCl4 system played a masterfully orchestrated dual role): 1) acylation of N-phenylethylenediamine with polyfluorinated carboxylic acid requiring all three reagents, 2) conversion of the resulting polyfluorinated carboxamide into the respective imidoyl chloride promoted by the PPh3–CCl4 dyad, and 3) spontaneous cyclization into imidazoline 109 requiring only triethylamine (Scheme 33).

Scheme. 33
Last year, Mosey and coworkers published62 a modern version of the cyclization of 2-chloroethylamidines into the respective 2-imidazolines described by Partridge in 1949.52 N-(2-Chloroethyl)benzamide was treated with triflic anhydride in pyridine which led to the formation of the N-imidoylpyridinium triflate intermediate 110. The latter could then be treated with various aromatic amines to form amidine 111 which cyclized spontaneously to give the target 2-imidazolines 112 (Scheme 34). The scope of (hetero)-aromatic groups that can be conveniently accommodated at the nitrogen atom of 2-imidazoline nucleus is remarkable and so are the yields (Fig. 14). Therefore, it is likely that this approach will nicely complement the metal-catalyzed versions12 , 24 , 25 to access compounds 112, particularly when it comes to the preparation of the most sterically congested analogs where using the Mosey protocol appears to be particularly advantageous.

Scheme. 34
Scope of N-(hetero)aryl-2-imidazolines 112 accessed as shown in Scheme 33.
In 2002, Glaxo scientists reported agonists of beta-3 adrenoreceptor (of particular utility as anti-obesity and anti-diabetes agents) exemplified by compound 113 which was prepared according to the synthetic route shown in Scheme 35 63 , 64 It involves the preparation of amidinium hydrochloride 114 which underwent spontaneous cyclization into imidazoline 113 when converted to free base.

Scheme. 35
In 2008, Korean scientists from Legochem Bioscience reported factor Xa inhibitors (exemplified by compound 115), potentially useful as anticoagulant drugs devoid of bleeding risk effects, which are related to marketed drug rivaroxaban (the latter belongs to the same class of anticlotting agents).65 The N-aryl-2-imidazoline moiety in compound 115 (serving as a biosteric replacement of morpholinone in rivaroxaban) was installed in a somewhat elaborate fashion starting from aniline 116. The latter was reductively alkylated with Boc-aminoacetaldehyde. Acylation of the resulting compound 117 afforded intermediate 118. The latter was treated with HCl to remove the Boc group and refluxing AcOH to form the 2-imidazoline cycle in the structure 115 (Scheme 36). Clearly, a single 2-imidazoline N-arylation step would significantly shorten

Scheme. 36
this route.
Bristol-Myers Squibb scientists described antiviral activity of 1H-pyrrolo[2,3-c]pyridine compounds containing imidazoline periphery (such as compound 119).66 The latter was synthesized as shown in Scheme 37. The route involves rather standard linear construction of the 2-imidazoline nucleus in the amide coupling partner 120. Again, this attests to the advantages of the recently reported convergent 2-imidazoline N-(hetero)arylation approaches.12 , 24 , 25

Scheme. 37
Interesting pteridine-based inhibitors of polo-like kinases containing N-aryl-2-imidazoline periphery were described by Elan Pharmaceuticals.67 , 68 The installation of the 2-imidazoline nucleus (shown for one of the examples, compound 121) involved a rare example of oxidative condensation of an N-(hetero)arylethylenediamine (in this case, compound 122) with benzaldehyde, a transformation widely used69 to prepare NH-imidazolines (Scheme 38).

Scheme. 38
The N-(hetero)aryl-2-imidazoline moiety was present in the synthetic and medicinal chemistry literature since the middle of the 20th century in the form of rather sporadic reports. Quite often the heterocycle in question was constructed using linear sequences. Until 2012, no dedicated methodological studies were undertaken to allow for independent variation of the periphery element around the N-(hetero)aryl-2-imidazoline core. The recently described metal-catalyzed and cyclocondensation methodologies attest to the resurgence of interest toward this heterocycle. Moreover, its privileged character in medicinal chemistry continues to be substantiated by emerging reports in the literature.
This research was supported by the Russian Scientific Foundation (project grant 14-50-00069).
References
Regunathan, S.; Reis, D. J. Ann. Rev. Pharmacol. Toxicol. 1996, 36, 511.
Krasavin, M. Eur. J. Med. Chem. 2015, 97, 525.
Gust, R.; Keilitz, R.; Schmidt, K. J. Med. Chem. 2001, 44, 1963.
Azevedo, L. M.; Lansdell, T. A.; Ludwig, J. R.; Mosey, R. A.; Woloch, D. K.; Cogan, D. P.; Patten, G. P.; Kuszpit, M. R.; Fisk, J. S.; Tepe, J. J. J. Med. Chem. 2013, 56, 5974.
Merriman, G. H.; Ma, L.; Shum, P.; McGarry, D.; Volz, F.; Sabol, J. S.; Gross, A.; Zhao, Z.; Rampe, D.; Wang, K.; Wirtz-Brugger, F.; Harris, B. A.; McDonald, D. Bioorg. Med. Chem. Lett. 2005, 15, 435.
Patel, S.; Player, M. R. Expert Opin. Invest. Drugs 2008, 17, 1865.
Evans, B. E.; Rittle, K. E.; Bock, M. G.; DiPardo, R. M.; Freidinger, R. M.; Whitter, W. L.; Lundell, G. F.; Veber, D. F.; Anderson, P. S.; Chang, R. S. L.; Lotti, V. J.; Cerino, D. J.; Chen, T. B.; Kling, P. J.; Kunkel, K. A.; Springer, J. P.; Hirshfeld, J. J. Med. Chem. 1988, 31, 2235.
Rauws, T. R. M.; Maes, B. U. W. Chem. Soc. Rev. 2012, 41, 2463.
Kantin, G.; Krasavin, M. Curr. Org. Chem. 2016, 20, 1370.
Hopkins, A. L.; Keserü, G. M.; Leeson, P. D.; Rees, D. C.; Reynolds, C. H. Nat. Rev. Drug Discovery 2014, 13, 105.
(a) Kort, M. E.; Drizin, I.; Gregg, R. J.; Scanio, M. J. C.; Shi, L.; Gross, M. F.; Atkinson, R. N.; Johnson, M. S.; Pacofsky, G. J.; Thomas, J. B.; Carroll, W. A.; Krambis, M. J.; Liu, D.; Shieh, C.-C.; Zhang, X.; Hernandez, G.; Mikusa, J. P.; Zhong, C.; Joshi, S.; Honore, P.; Roeloffs, R.; Marsh, K. C.; Murray, B. P.; Liu, J., Werness, S.; Faltynek, C. R.; Krafte, D. S.; Jarvis, M. F.; Chapman, M. L.; Marron, B. E. J. Med. Chem. 2008, 51, 407. (b) Renton, P.; Green, B.; Maddaford, S.; Rakhit, S.; Andrews, J. S. ACS Med. Chem. Lett. 2012, 3, 227.
Krasavin, M. Tetrahedron Lett. 2012, 53, 2876.
Chuprina, A.; Lukin, O.; Demoiseaux, R.; Buzko, A.; Shivanyuk, A. J. Chem. Inf. Model. 2010, 50, 470.
Murray, C. W.; Rees, D. C. Nat. Chem. 2009, 1, 187.
Pesson, M.; Antoine, M.; Girard, P.; Chabassier, S.; Richer, D.; Benichon, J. L.; De Lajudie, P.; Horvath, E.; Leriche, B.; Patte, S. Eur. J. Med. Chem. 1975, 10, 567; Chem. Abstr. 1976, 84, 164712.
Mailliet, P.; Laoui, A.; Riou, J.-F.; Doerflinger, G.; Mergny, J.-L.; Hamy, F.; Caulfield, T. WO Patent 2002076975; Chem. Abstr. 2002, 137, 263071.
Caroff, E.; Fretz, H.; Hilpert, K.; Houille, O.; Hubler, F.; Meyer, E. WO Patent 2006114774; Chem. Abstr. 2006, 145, 471852.
Ashwell, M. A.; Liu, Y.; Ali, S.; Hill, J.; Wrona, W. WO Patent 2006010082; Chem. Abstr. 2006, 144, 150384.
Ryu, J. M.; Lee, J. S.; Park, W. J.; Hwang, Y. H.; Kim, K. Y. WO Patent 2010067987; Chem. Abstr. 2010, 153, 62137.
Bamberg, J. T.; Bartlett, M.; Dubois, D. J.; Elworthy, T. R.; Hendricks, R. T.; Hermann, J. C.; Kondru, R. K.; Lemoine, R.; Lou, Y.; Owens, T. D.; Park, J.; Smith, D. B.; Soth, M.; Yang, H.; Yee, C. W. US Patent 20090215750; Chem. Abstr. 2009, 151, 313587.
Clayton, J.; Ma, F.; Van Wagenen, B.; Ukkiramapandian, R.; Egle, I.; Empfield, J.; Isaac, M.; Slassi, A.; Steelman, G.; Urbanek, R.; Walsh, S. WO Patent 2006020879; Chem. Abstr. 2006, 144, 254003.
Calculated using software provided by Molinspiration Cheminformatics, Slovensky Grob, Slovak Republic. http://www.molinspiration.com.
Congreve, M.; Carr, R.; Murray, C.; Jhoti, H. Drug Discovery Today 2003, 8, 876.
Davis, O. A.; Hughes, M.; Bull, J. A. J. Org. Chem. 2013, 78, 3470.
Dar'in, D.; Krasavin, M. J. Org. Chem. 2016, 81, 12514.
Elliott, M. L.; Welch, W. M. WO Patent 9743276; Chem. Abstr. 1997, 128, 34774.
Penning, T. D.; Talley, J. J.; Bertenshaw, S. R.; Carter, J. S.; Collins, P. W.; Docter, S.; Graneto, M. J.; Lee, L. F.; Malecha, J. W.; Miyashiro. J. M.; Rogers, R. S.; Rogier, D. J.; Yu, S. S.; Anderson, G. D.; Burton, E. G.; Cogburn, J. N.; Gregory, S. A.; Koboldt, C. M.; Perkins, W. F.; Seibert, K.; Veenhuizen, A. W.; Zhang, Y. Y.; Isakson, P. C. J. Med. Chem. 1997, 40, 1347.
Khanna, I. K.; Weier, R. M.; Yu, Y.; Xu, X. D.; Koszyk, F. J.; Collins, P. W.; Koboldt, C. M.; Veenhuizen, A. W.; Perkins, W. E.; Casler, J. J.; Masferrer, J. L.; Zhang, Y. Y.; Gregory, S. A.; Seibert, K.; Isakson, P. J. Med. Chem. 1997, 40, 1634.
Krasavin, M. Lett. Org. Chem. 2013, 10, 235.
Michaux, C.; Charlier, C. Mini-Rev. Med. Chem. 2004, 4, 603.
Sarnpitak, P.; Mujumdar, P.; Morisseau, C.; Hwang, S. H.; Hammock, B.; Iurchenko, V.; Zozulya, S.; Gavalas, A.; Geronikaki, A.; Ivanenkov, Y.; Krasavin, M. Eur. J. Med. Chem. 2014, 84, 160.
Yoshino, T.; Kimoto, A.; Kobayashi, S.; Noguchi, M.; Fukunaga, M.; Hayashi, A.; Miyata, K.; Sasamata, M. Arzneim. Forsch. 2005, 55, 394.
Supuran, C.T.; Kalinin, S.; Tanē, M.; Sarnpitak, P.; Mujumdar, P. Poulsen, S.-A.; Krasavin, M. J. Enzyme Inhib. Med. Chem. 2016, 31(S1), 197.
Supuran, C. T. J. Enzyme Inhib. Med. Chem. 2016, 31, 345.
Krasavin, M.; Mujumdar, P.; Parchinsky, V.; Vinogradova, T.; Manicheva, O.; Dogonadze, M. J. Enzyme Inhib. Med. Chem. 2016, 31, 1146.
Smilkstein, M.; Sriwilaijaroen, N.; Kelly, J. X.; Wilairat, P.; Riscoe, M. Antimicrob. Agents Chemother. 2004, 48, 1803.
Zhao, H.; Dong, J.; Lafleur, K.; Nevado, C.; Caflisch, A. ACS Med. Chem. Lett. 2012, 3, 834.
Sarnpitak, P.; Mujumdar, P.; Taylor, P.; Cross, M.; Coster, M. J.; Gorse, A.-D.; Krasavin, M.; Hofmann, A. Biotechnol. Adv. 2015, 33, 941.
Mujumdar, P.; Sarnpitak, P.; Shetnev, A.; Dorogov, M.; Krasavin, M. Tetrahedron Lett. 2015, 56, 2827.
Urich, R.; Wishart, G.; Kiczun, M.; Richters, A.; Tidten-Luksch, N.; Rauh, D.; Sherborne, B.; Wyatt, P. G.; Brenk, R. ACS Chem. Biol. 2013, 8, 1044.
Mujumdar, P.; Grkovic, T.; Krasavin, M. Tetrahedron Lett. 2013, 54, 3336.
Han, C.; Zhang, J.; Zheng, M.; Xiao, Y.; Li, Y.; Liu, G. Mol. Diversity 2011, 15, 857.
Chytil, M.; Engel, S. R.; Fang, Q. K.; Spear, K. L. US Patent 20100204214A1; Chem. Abstr. 2010, 153, 311281.
Deng, W.; Chen, D.; Zhou, Y. WO Patent 2006101456A1; Chem. Abstr. 2006, 145, 1009636.
Kon-I, K.; Matsumizu, M.; Shima, A. US Patent 20060094750A1; Chem. Abstr. 2006, 144, 409719.
Korshin, E. E.; Sabirova, L. I.; Akhmadullin, A. G.; Levin, Ya. A. Russ. Chem. Bull. 1994, 43, 431. [Izv. Akad. Nauk, Ser. Khim. 1994, 472.]
Mujumdar, P.; Korsakov, M.; Dorogov, M.; Krasavin, M. Synlett 2014, 2323.
Karamysheva, K.; Reutskaya, E.; Sapegin, A.; Dorogov, M.; Krasavin, M. Tetrahedron Lett. 2015, 56, 5632.
Sapegin, A. V.; Kalinin, S. A.; Smirnov, A. V.; Dorogov, M. V.; Krasavin, M. Eur. J. Org. Chem. 2015, 1333.
Sapegin, A.; Osipyan, A.; Krasavin, M. Org. Biomol. Chem. 2017, 15, 2906.
Bauer, R. A.; Wenderski, T. A.; Tan, D. S. Nat. Chem. Biol. 2013, 9, 21.
Partridge, M. W.; Turner, H. A. J. Chem. Soc. 1949, 1308.
Clayton, G. C. Ber. Dtsch. Chem. Ges. 1895, 28, 1665.
Heine, H. W.; Bender, H. S. J. Org. Chem. 1960, 25, 461.
Khlebnikov, A. F.; Novikov, M. S.; Petrovskii, P. P.; Stoeckli-Evans, H. J. Org. Chem. 2011, 76, 5384.
Seelinger, W.; Aufderhaar, E.; Diepers, W.; Feinauer, R.; Nehring, R.; Thier, W.; Hellmann, H. Angew. Chem. 1966, 78, 913.
Partridge, M. W.; Smith, A. J. Chem. Soc., Perkin Trans. 1 1973, 453.
García, B.; Torres, R. A.; Orelli, L. R. Tetrahedron Lett. 2006, 47, 4857.
Reverdito, A. M.; Perillo, I. A.; Salerno, A. Synth. Commun. 2012, 42, 2083.
Lakner, F. J.; Parker, M. A.; Rogovoy, B.; Khvat, A.; Ivachtchenko, A. Synthesis 2009, 1987.
Jiang, H.; Sun, L.; Yuan, S.; Lu, W.; Wan, W.; Zhu, S.; Hao, J. Tetrahedron 2012, 68, 2858.
Ellsworth, A. A.; Magyar, C. L.; Hubbell, G. E.; Chelsea, C. T.; Holmes, D.; Mosey, R. A. Tetrahedron 2016, 72, 6380.
Cooke, J. W. B.; Glover, B. N.; Lawrence, R. M.; Sharp, M. J.; Tymoschenko, M. F. WO Patent 2002066418; Chem. Abstr. 2002, 137, 185304.
Deaton, D. N.; Shearer, B. G.; Uehling, D. E. WO Patent 2002060885; Chem. Abstr. 2002, 137, 154843.
Cho, Y. L.; Song, H. Y.; Lee, D. Y.; Baek, S. Y.; Chae, S. E.; Jo, S. H.; Kim, Y. O.; Lee, H. S.; Park, J. H.; Park, T. K.; Woo, S. H.; Kim, Y. Z. WO Patent 2008140220; Chem. Abstr. 2008, 149, 576540.
Wang, T.; Kadow, J. F.; Zhang, Z.; Yin, Z.; Meanwell, N. A.; Regueiro-Ren, A.; Swidorski, J.; Han, Y.; Carini, D. J.; Hamann, L. G. US Patent 20080139572; Chem. Abstr. 2008, 149, 54018.
Galemmo, R. A.; Artis, D. R.; Ye, X. M.; Aubelle, D. L.; Truong, A. P.; Bowers, S.; Hom, R. K.; Zhu, Y.-L.; Neitz, R. J.; Sealy, J.; Adler, M.; Beroza, P.; Anderson, J. P. WO Patent 2011079114; Chem. Abstr. 2011, 155, 152557.
Neitz, R. J.; Troung, A. P.; Galemmo, R. A.; Ye, X. M.; Sealy, J.; Adler, M.; Bowers, S.; Beroza, P.; Anderson, J. P.; Aubele, D. L.; Artis, D. R.; Hom, R. K.; Zhu, Y. WO Patent 2012048129; Chem. Abstr. 2012, 156, 505554.
Gogoi, P.; Konwar, D. Tetrahedron Lett. 2006, 47, 79.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2017, 53(3), 240–255
Rights and permissions
About this article
Cite this article
Krasavin, M. N-(Hetero)aryl-2-imidazolines: an emerging privileged motif for contemporary drug design. Chem Heterocycl Comp 53, 240–255 (2017). https://doi.org/10.1007/s10593-017-2047-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-017-2047-3