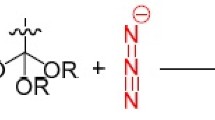This review presents current data (2007–2015) on the methods of synthesis of tetrazole-5-thiones and their S-functionalization.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
 In the last decade, tetrazoles have attracted the attention of chemists thanks to a variety of applications in medicine,1 agriculture,2 as well as energetic materials3 and ligands to produce stable complexes.4 Tetrazole-5-thiones are of particular interest. The presence of an additional reaction center (sulfur atom) in the structure of tetrazole-5-thiones makes them suitable synthons for accessing a number of novel compounds and materials. Thus, heterocyclic sulfones, obtained by oxidation of tetrazolyl sulfides, are widely used in modern synthetic organic chemistry for the selective and efficient olefination of aldehydes by the Julia–Kocienski method.5 For example, using 1-(tert-butyl)-tetrazolyl sulfone in reactions of this type affords Z-trisubstituted alkenes with high efficiency, avoiding the separation of diastereomers.5b Whereas chemistry of tetrazoles as a whole has been the focus of literature reviews,6 tetrazolethiones have not yet found detailed coverage in review literature.
In the last decade, tetrazoles have attracted the attention of chemists thanks to a variety of applications in medicine,1 agriculture,2 as well as energetic materials3 and ligands to produce stable complexes.4 Tetrazole-5-thiones are of particular interest. The presence of an additional reaction center (sulfur atom) in the structure of tetrazole-5-thiones makes them suitable synthons for accessing a number of novel compounds and materials. Thus, heterocyclic sulfones, obtained by oxidation of tetrazolyl sulfides, are widely used in modern synthetic organic chemistry for the selective and efficient olefination of aldehydes by the Julia–Kocienski method.5 For example, using 1-(tert-butyl)-tetrazolyl sulfone in reactions of this type affords Z-trisubstituted alkenes with high efficiency, avoiding the separation of diastereomers.5b Whereas chemistry of tetrazoles as a whole has been the focus of literature reviews,6 tetrazolethiones have not yet found detailed coverage in review literature.

 Elena Sergeevna Il'inykh was born in 1987 in Chelyabinsk, Russia. She graduated from the Chelyabinsk State University in 2009, in 2013 she defended her thesis at the Ural Federal University. She is currently Associate professor of organic chemistry at the South Ural State University (SUSU). Her research interests include chemistry of heterocyclic compounds, materials for organic electronics.
Elena Sergeevna Il'inykh was born in 1987 in Chelyabinsk, Russia. She graduated from the Chelyabinsk State University in 2009, in 2013 she defended her thesis at the Ural Federal University. She is currently Associate professor of organic chemistry at the South Ural State University (SUSU). Her research interests include chemistry of heterocyclic compounds, materials for organic electronics.
 Dmitry Gymnanovich Kim was born in 1948 in Pskent, Uzbekistan. He graduated from the Faculty of Chemistry of the Irkutsk State University (ISU) in 1972, from 1972 to 1975, he studied in graduate school at A. E. Favorsky Irkutsk Institute of Organic Chemistry. In 1977 he defended his candidate thesis (ISU), while in 2004 his doctoral thesis (Ural State Technical University). Currently he is head of the Department of organic chemistry, SUSU, D.Sci., Professor. His research interests include chemistry of heterocyclic compounds, halocyclization reactions. He is the author of 170 publications, including 4 reviews in Chemistry of Heterocyclic Compounds.
Dmitry Gymnanovich Kim was born in 1948 in Pskent, Uzbekistan. He graduated from the Faculty of Chemistry of the Irkutsk State University (ISU) in 1972, from 1972 to 1975, he studied in graduate school at A. E. Favorsky Irkutsk Institute of Organic Chemistry. In 1977 he defended his candidate thesis (ISU), while in 2004 his doctoral thesis (Ural State Technical University). Currently he is head of the Department of organic chemistry, SUSU, D.Sci., Professor. His research interests include chemistry of heterocyclic compounds, halocyclization reactions. He is the author of 170 publications, including 4 reviews in Chemistry of Heterocyclic Compounds.
Synthesis of tetrazole-5-thiones
 The traditional approach to the synthesis of tetrazolethiones, first proposed by Lieber et al. in 1957,7 is based on the reaction of isothiocyanates with NaN3 in water or ethanol. Significant disadvantages of this method are the limited number of suitable isothiocyanates, severe reaction conditions (heating for 4–8 h), and low yields.
The traditional approach to the synthesis of tetrazolethiones, first proposed by Lieber et al. in 1957,7 is based on the reaction of isothiocyanates with NaN3 in water or ethanol. Significant disadvantages of this method are the limited number of suitable isothiocyanates, severe reaction conditions (heating for 4–8 h), and low yields.
The use of pyridine as the base in this reaction leads to the completion of the process already after 2 h at room temperature to give 1-substituted 1H-tetrazole-5-thiones in 72–99% yields.8 Also provided is a convenient and efficient one-pot method for the synthesis of 1-substituted 5-alkylsulfanyl-1H-tetrazoles from isothiocyanates and NaN3 followed by addition of alkyl halides.


Synthesis of tetrazole-5-thiones (continued)  Substitution of isothiocyanates with arylthiocyanates in the reaction with NaN3 allows for an easy one-step synthesis of S-aryl derivatives of 1H-tetrazole-5-thiones. Thus, 2-amino-4,6-dimethoxy-5-(1H-tetrazol-5-ylsulfanyl)pyrimidine was obtained by reacting thiocyanatopyrimidine with NaN3 in the presence of NH4Cl in DMF.9
Substitution of isothiocyanates with arylthiocyanates in the reaction with NaN3 allows for an easy one-step synthesis of S-aryl derivatives of 1H-tetrazole-5-thiones. Thus, 2-amino-4,6-dimethoxy-5-(1H-tetrazol-5-ylsulfanyl)pyrimidine was obtained by reacting thiocyanatopyrimidine with NaN3 in the presence of NH4Cl in DMF.9
Haidar et al. have developed a new technique for the synthesis of 1-aryl(alkyl)-1H-tetrazole-5-thiones from tetramethylthiuram disulfide via isolation of intermediate 3-substituted 1,1-dimethylthiourea.10
An alternative method of preparing tetrazolethiones involves the use of tetrazol-5-ones as precursors. A series of 1-substituted 4-methyl-1H-tetrazole-5-thiones was obtained by the action of P2S5 on the corresponding 4-methyl-1Htetrazol-5-ones.11 Lawesson's reagent is also used as a thiocarbonylating agent in such reactions.12


Alkylation
 It is known that tetrazolethiones are ambident nucleophiles. However, in alkylation reactions with different alkyl halides they act solely as S-nucleophiles.1b,
13 Thus, a series of 1-alkyl/aryl-5-benzylsulfanyl-1H-tetrazoles was synthesized via a reaction of 1-substituted 1H-tetrazole-5-thiones with the corresponding benzyl halides under phase-transfer catalysis conditions or in acetonitrile in the presence of Et3N.1b
It is known that tetrazolethiones are ambident nucleophiles. However, in alkylation reactions with different alkyl halides they act solely as S-nucleophiles.1b,
13 Thus, a series of 1-alkyl/aryl-5-benzylsulfanyl-1H-tetrazoles was synthesized via a reaction of 1-substituted 1H-tetrazole-5-thiones with the corresponding benzyl halides under phase-transfer catalysis conditions or in acetonitrile in the presence of Et3N.1b

The reaction of N-boryltetrazolethione with benzyl bromides by heating in acetonitrile similarly proceeds regiospecifically to form the S-alkylation products.13b

Only further alkylation of 5-aryl/benzylsulfanyl-1H-tetrazoles with alkyl halides leads to the corresponding N-derivatives.9 , 14 5-Benzylsulfanyl-1-cyclopentyl-1H-tetrazole was synthesized by the action of cyclopentyl bromide on benzylsulfanyltetrazole by heating in DMF in the presence of K2CO3.14

A fundamentally new, simple, convenient one-pot method for the synthesis of alkyl(1H-tetrazol-5-yl)thioethers has been developed, comprising condensation of aldehydes with N-tosylhydrazine to produce tosylhydrazones, which are then used in situ in reactions with 1H-tetrazole- 5-thiones.15


Arylation
 Methods for the synthesis of 9-alkyl-6-(1-methyl-1H-tetrazol-5-ylsulfanyl)-9H-purines16 and 2-ethoxy-3,5,6-trifluoro-4-(1-methyl-1H-tetrazol-5-ylsulfanyl)pyridine17 have been proposed based on the reaction of 1-methyl-1H-tetrazol-5-thione with respectively 9-alkyl-6-chloro-9H-purines or pentafluoropyridine.
Methods for the synthesis of 9-alkyl-6-(1-methyl-1H-tetrazol-5-ylsulfanyl)-9H-purines16 and 2-ethoxy-3,5,6-trifluoro-4-(1-methyl-1H-tetrazol-5-ylsulfanyl)pyridine17 have been proposed based on the reaction of 1-methyl-1H-tetrazol-5-thione with respectively 9-alkyl-6-chloro-9H-purines or pentafluoropyridine.


Reactions with chloroethynylphosphonates
 The reaction of 1-substituted 1H-tetrazole-5-thiones with an equimolar amount of dialkyl chloroethynylphosphonates in acetonitrile proceeds ambiguously. According to 1H, 13C, and 31P NMR, the major reaction product is the cyclic thiazolotetrazole zwitterion, whereas the phosphonate and thioethynylphosphonate are formed in negligible amounts.18
The reaction of 1-substituted 1H-tetrazole-5-thiones with an equimolar amount of dialkyl chloroethynylphosphonates in acetonitrile proceeds ambiguously. According to 1H, 13C, and 31P NMR, the major reaction product is the cyclic thiazolotetrazole zwitterion, whereas the phosphonate and thioethynylphosphonate are formed in negligible amounts.18


References
(a) Romagnoli, R.; Baraldi, P. G.; Salvador, M. K.; Preti, D.; Tabrizi, M. A.; Brancale, A.; Fu, X.-H.; Li, J.; Zhang, S.-Z.; Hamel, E.; Bortolozzi, R.; Basso, G.; Viola, G. J. Med. Chem. 2012, 55, 475. (b) Karabanovich, G.; Roh, J.; Smutný, T.; Němeček, J.; Vicherek, P.; Stolaříková, J.; Vejsová, M.; Dufková, I.; Vávrová, K.; Pávek, P.; Klimešová, V.; Hrabálek, A. Eur. J. Med. Chem. 2014, 82, 324. (c) Wei, C.-X.; Bian, M.; Gong, G.-H. Molecules 2015, 20, 5528.
Luo, Y.-P.; Yang, G.-F. Bioorg. Med. Chem. 2007, 15, 1716.
(a) Srinivas, D.; Ghule, V. D.; Muralidharan, K.; Jenkins, H. D. B. Chem.–Asian J. 2013, 8, 1023. (b) Srinivas, D.; Ghuleb, V. D.; Muralidharan, K. RSC Adv. 2014, 4, 7041. (c) Fischer, D.; Klapötke, T. M.; Stierstorfer, J. Angew. Chem., Int. Ed. 2015, 54, 10299.
(a) Yu, Q.; Huang, F.-P.; Yang, Z.-M.; Jin, J.; Bian, H.-D.; Liang, H. Polyhedron 2012, 33, 203. (b) Tafili-Kryeziua, M.; Caneschib, A.; Fittipaldic, M.; Spinac, G.; Lantierid, M.; Weile, M.; Hasegawaf, M.; Linert, W. J. Coord. Chem. 2015, 68, 3457.
(a) Singh, G.; Kumar, R.; Swett, J.; Zajc, B. Org. Lett. 2013, 15, 4086. (b) Lorente, A.; Albericio, F.; Álvarez, M. J. Org. Chem. 2014, 79, 10648.
(a) Ostrovskii, V. A.; Koldobskii, G. I.; Trifonov, R. E. In Comprehensive Heterocyclic Chemistry III; Katritzky, A. R., Ed.; Elsevier: New York, 2008, Vol. 6, p. 257. (b) Yet, L. In Progress in Heterocyclic Chemistry; Gribble, G. W.; Joule, J., Eds.; Pergamon: Oxford, 2011, Vol. 23, p. 231.
(a) Lieber, E.; Pillai, C. N.; Hites, R. D. Can. J. Chem. 1957, 35, 832. (b) Lieber, E.; Ramachahdran, J. Can. J. Chem. 1959, 37, 101.
Han, S. Y.; Lee, J. W.; Kim, H.-J.; Kim, Y.-J.; Lee, S. W.; Gyoung, Y. S. Bull. Korean Chem. Soc. 2012, 33, 55.
Dişli, A.; Mercan, S.; Yavuz, S. J. Heterocycl. Chem. 2013, 50, 1446.
Haidar, Saaod; Severina, A. I.; Georgiyants, Actual Questions of Pharmaceutical and Medical Science and Practice [in Ukranian] 2013, 2(12), 18.
Rayat, S.; Chhabra, R.; Alawode, O.; Gundugola, A. S. J. Mol. Struct. 2009, 933, 38.
Gundugola, A. S.; Chandra, K. L.; Perchellet, E. M.; Waters, A. M.; Perchellet, J.-P. H.; Rayat, S. Bioorg. Med. Chem. Lett. 2010, 20, 3920.
(a) Zhang, Q.; Zhang, H.; Wang, J.; Zhang, C.; Sun, D. Heteroat. Chem. 2012, 23, 435. (b) Pan, X.; Curran, D. P. Org. Lett. 2014, 16, 2728.
Saglam, S.; Disli, A.; Erdogdu, Y.; Marchewka, M. K.; Kanagathara, N.; Bay, B.; Güllüoğlu, M. T. Spectrochim. Acta, Part A 2015, 135, 1011.
Li, L.-L.; Gao, L.-X.; Han, F.-S. RSC Adv. 2015, 5, 29996.
Celik, G. D.; Disli, A.; Oner, Y.; Acik, L. Med. Chem. Res. 2013, 22, 1470.
Beyki, K.; Haydari, R.; Maghsoodlou, M. T. SpringerPlus 2015, 4, 757.
(a) Erkhitueva, E. B.; Egorov, D. M.; Dogadina, A. V.; Khramchikhin, A. V.; Ionin, B. I. Russ. J. Gen. Chem. 2012, 82, 2011. [Zh. Obshch. Khim. 2012, 2059.] (b) Erkhitueva, E. B.; Dogadina, A. V.; Khramchikhin, A. V.; Ionin, B. I. Tetrahedron Lett. 2013, 54, 5174.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2016, 52(6), 356–358
Rights and permissions
About this article
Cite this article
Il’inykh, E.S., Kim, D.G. Tetrazolethiones: synthesis and S-functionalization reactions (microreview). Chem Heterocycl Comp 52, 356–358 (2016). https://doi.org/10.1007/s10593-016-1894-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-016-1894-7