The reactions of 4-chloro-5,7-dinitro-4-benzofurazan with indole and pyrrole derivatives, occurring by SNAr–SEAr mechanism, led to the formation of dihetaryls with intramolecular charge transfer. A method was developed for the annelation of pyrrole and dihydropyrrole ring to nitrobenzofurazan fragment by adding unstabilized azomethine ylide to the С=С bond of dinitrobenzofurazan. The structure of nitrobenzofurazan derivatives was studied by X-ray structural analysis, NMR spectroscopy, and quantum-chemical calculations using ab initio and DFT methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
4,6-Dinitrobenzofuroxan (1a) and 4,6-dinitrobenzofurazan (2a) are superelectrophilic [1, 2] heteroaromatic structures that interact with N-, O-, and S-nucleophiles, forming highly stable anionic [3–5] and bipolar spirocyclic Meisenheimer σ-complexes [6–9], as well as intramolecular π-stacked charge transfer complexes [10]. We have previously established that chloro derivatives 1b and 2b react under mild conditions with π-electron rich nitrogen heterocycles (pyrroles, indoles, indolizines), forming dihetaryls 3 (in the case of reaction with indolizines) [11–13].
The structure and characteristic reactions of neutral aromatic superelectrophiles – nucleophilic substitution and addition, Diels–Alder reactions with normal and inverse electron demand, Michael addition, and recyclization involving the N-oxide oxygen atom, as well as experimental methods for quantitative characterization of electrophilicity – are considered in a monograph [14], reviews [15–18], and references therein. The analysis of these literature data shows that reactivity of dinitrobenzofurazan derivatives remains largely unexplored and underestimated, compared to dinitrobenzofuroxan derivatives. In our view, this is a consequence of difficulties in preparative synthesis of both 4,6-dinitrobenzofurazan (2a) and its chloro derivative 2b. We should note here that the lack of N-oxide oxygen atom allows to avoid N1 ⇄ N3 oxide tautomerism and Boulton–Katritzky type rearrangements [1], which makes the superelectrophile 2 a more convenient object for the study of nucleophilic substitution and cycloaddition reactions.

In this work, we report the synthesis and structure of new indole and pyrrole derivatives containing a dinitrobenzofurazan fragment, as well as [3+2] cycloaddition of unstabilized azomethine ylides to these compounds.
The choice of specifically indolyl and pyrrolyl derivatives as dipolarophiles was motivated by two basic reasons. First of all, it is known that both С=С bonds of the six-membered ring in nitrobenzoxadiazoles 1a and 2a show good reactivity in cycloaddition reactions [15, 16]. The introduction of bulky electron-donating substituent at position 7 sharply increases the regioselectivity of cycloaddition and directs the attack by azomethine ylide exclusively at the С(6)=С(7) bond. In addition, nitrobenzoxadiazoles are frequently recognized as potential lead compounds for development of drugs that serve as exogenous sources of nitric oxide (NO). The presence of additional pharmacophoric indole or pyrrole fragments in the molecules of nitrobenzoxadiazoles thus increases their potential value in medicinal chemistry.
The interaction between equivalent amounts of 4-chloro-5,7-dinitrobenzofurazan (2b) and 1,2-di-methylindole (4) in chloroform at room temperature produced a high yield of dihetaryl 6, possessing an intense blue color.

The formation of a new carbon–carbon bond occurred through an S NAr–S EAr reaction, involving the bipolar Meisenheimer–Wheland σ-complex 5 as intermediate. We had previously established the mechanism of analogous reaction between electrophile 1b and N-methylindole by kinetic experiments, including the use of deuterated indole derivatives [11]. An additional evidence was obtained by preparative isolation of σ-complex, corresponding to structure 5, which was obtained by interaction of electrophile 1a and 1,3,5-tris(piperidin-1-yl)-benzene [19].
We performed a reaction of dihetaryl 6 with the nonstabilized azomethine ylide 9, obtained in situ from sarcosine (7) and formaldehyde (8).
The results of reactions depended on the solvents used. Heating a mixture of dihetaryl 6, sarcosine (7), and paraform in benzene gave the cycloaddition product 10 in 94% yield. When MeCN was used instead of benzene, the initially also formed cycloadduct 10 likely underwent elimination of HNO2 during the reaction, forming the dihydropyrrole 11, followed by oxidative aromatization to pyrrole derivative 12. The reaction mixture was separated by column chromatography, the yields of products 11 and 12 were 29 and 5%, respectively. It is important to note that the 1Н NMR spectrum of cycloadduct 10 contained two groups of signals in 3:2 ratio, with similar chemical shifts and practically identical spin-spin coupling parameters.

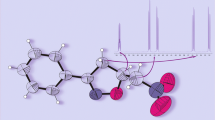
In order to identify likely reasons for the formation of two stereoisomers, we performed X-ray structural analysis of dihetaryl 6, as well as quantum-chemical calculations of its geometrical parameters within the framework of density functional theory (DFT). The structure of compound 6 is presented in Figure 1, while its main geometrical characteristics are given in Table 1.
Steric hindrance in the molecule of compound 6 prevents a planar conformation – the dihedral angle between planes of indole and furazan fragments is 46° according to DFT calculations (also 46° acccording to X-ray structural analysis), which may cause atropisomerism. The nitro group at position 5 must be rotated by 32° relative to the furazan ring plane according to calculations for MeCN solution (43° according to X-ray structural analysis). Thus, the distance between the nitro group O(3) oxygen and С(6) carbon atoms was 2.70 Å (2.74 Å according to X-ray structural analysis), while the distance between the other oxygen atom, O(4), of nitro group and the С(6) atom was 3.48 Å (3.43 Å according to X-ray structural analysis data).
Polar cycloaddition to the asymmetric multiple bond is a concerted, albeit asynchronous process, and starts from nucleophilic attack by carbon atom at the most electrophilic carbon atom С(6) [17, 18]. This means that differences in steric accessibility of the С(6) carbon atom for attack by azomethine ylide from different sides of benzofurazan ring plane may affect both the rate of cycloaddition, as well as the stability of the obtained isomeric cycloadducts. The reaction pathway where oxygen atom is located farther from С(6) atom, and thus presents lower steric obstacles to the approach by azomethine ylide, is favored more than the alternative attack from the other side of benzofurazan ring plane. In other words, the approach of azomethine ylide to the diastereofacial plane of benzofurazan ring (from top or bottom) is no longer equally probable, and thus lead to the observed ratio of stereoisomers 10a and 10b.
This assumption was also supported by ab initio quantum-chemical calculations of stereoisomeric cycloadducts 10a,b in gas phase, using 6-31G** basis set. Thus, attack from the side of nearby oxygen atom O(3) gave product 10b, less stable by 2.0 kcal/mol than product 10a, formed by attack from the side of the more distant oxygen atom O(4). The computed structures of stereoisomeric cycloaddition products are presented in Figure 2.
The non-coplanar arrangement of benzofurazan and indole fragments was also manifested as a non-equivalence of geminal protons in dihydropyrrole ring of compound 11. Each of the methylene proton pairs gave diastereotopic signals in the 1Н NMR spectra, indicating that the molecule was chiral. The chirality can be explained by restricted rotation of the two fragments in molecule 11 around the С(4)–С(9) bond, because there is no asymmetric carbon atom in the molecule of cycloadduct after elimination of HNO2.
The reaction of dinitrochlorobenzofurazan 2b with N-benzylpyrrole (13) in MeCN at room temperature gave the α- and β-pyrrolyl derivatives 14 and 15.

Each of the regioisomers was isolated by preparative column chromatography and characterized: compound 14 was obtained as violet rhombic crystals in 67% yield, while compound 15 as orange needles in 11% yield. All protons of pyrrole ring are known to be coupled, while the coupling constants have quite similar absolute values (1.3-3.5 Hz) [20], complicating reliable assignment of 1H NMR signals due to isomers 14 and 15. The 1H NMR spectra of regioisomers were interpreted based on NOESY experiments, taking into account the presence or absence of correlation between the methylеne protons of benzyl substituent and the α-protons of pyrrole ring. The structure of the minor reaction product was conclusively proved by X-ray structural analysis and is presented in Figure 3.
The molecule of compound 15 was practically planar, the dihedral angle between pyrrole and benzofurazan fragment planes was 3.6°. The characteristic bond lengths are given in Table 2. In our opinion, these bond lengths indicate a certain contribution of resonance structure with intramolecular charge transfer to the electron density distribution, as presented in Figure 3. The N(6)–C(7) bond in dinitrobenzofurazan fragment was shorter than the N(5)–C(5) bond. The pyrrole ring C(10)–C(11) and N(4)–C(8) bonds were likewise predictably shorter compared to C(8)–C(9) and N(4)–C(11) bonds. The C(4)–C(9) bond binding two hetaryl fragments was shorter than the standard С(Ar)–С(Ar) bond (1.50 Å) [21].
The C(5)–C(6) bond was slightly shortened, similarly as in the bipolar spirocyclic σ-complex based on dinitrobenzofurazan (1.390 Å) [9], compared to the analogous bond in dinitrobenzofuroxan 1a (1.44 Å) [22].
According to quantum-chemical calculations by DFT method, the charge transfer between the donor and acceptor fragments in molecule 15 was equal to 0.26 ē.
We introduced the major isomer 14 in cycloaddition reaction with azomethine ylide 9. Similarly as in the case of indole derivative 6, two cycloaddition products 16 and 17 were formed in the reaction in 30% and 3% yields, respectively. No cycloadduct with conserved nitro group (analogous to the indole derivative 10) could be isolated even when the reaction was performed in benzene

The structure of dihydropyrrole 16 was determined by X-ray structural analysis and is given in Figure 4. Unlike in the derivative 15, the pyrrole and benzofurazan fragments of dihydropyrrole 16 were not coplanar, forming a dihedral angle of 46°. The nitro group was also rotated relative to the plane of six-membered ring by 65°.
At the same time, the fully aromatized product 17 contained two π-electron rich pyrrole rings and one π-electron deficient nitrobenzofurazan fragment, and could be considered as donor–acceptor–donor (D–A–D) triad. The extent of charge transfer from the annelated and σ-bonded pyrrole ring to the nitrobenzofurazan fragment of molecule 17 was studied by quantum-chemical calculations according to DFT method. The N-benzylpyrrole fragment was found to be a weaker donor of electron density (0.08 ē) compared to the annelated N-methylpyrrole fragment (0.21 ē).
Thus, our developed method for the synthesis of С-substituted derivatives of dinitrobenzofurazan in combination with [3+2] cycloaddition reactions allowed to obtain new heterocyclic systems containing π-electron rich and π-electron deficient fragments, potentially of interest for medicinal chemistry [23] as exogenic donors of nitric oxide (NO) – a unique mediator of many physiological and pathological processes in the human body. In our opinion, the most likely NO source is 4-(1,2-dimethyl-1H-indol-3-yl)-7-methyl-5,8а-di-nitro-6,7,8,8а-tetrahydro-5аН-[1, 2, 5]oxadiazolo[3,4-е]isoindole obtained by us, characterized by low stability in solutions and the tendency towards spontaneous elimination of HNO2 molecule, serving as precursor to nitric oxide. Variation of π-electron rich fragments in molecules analogous to this compound would allow to design its spatial structure and to increase or decrease its complementarity with binding sites of principal biological targets (enzymes, membrane receptors, ion channels).
Experimental
1Н NMR spectra were acquired on a Bruker DPX-250 spectrometer (250 MHz) in acetone-d6 (compound 6) or in CDCl3 (the rest of compounds), internal standard was TMS. The high-resolution mass spectra were recorded on a Bruker micrOTOF II instrument with electrospray ionization. Measurements were performed in positive ion mode (capillary voltage 4500 V). Mass scanning range was 50-3000 Da. Melting points were determined in glass capillaries on a PTP apparatus. Column chromatography was performed with Merck silica gel 60 (70-230 μm). 4-Chloro-5,7-dinitrobenzofurazan (2b) was synthesized by a published procedure [24].
4-(1,2-Dimethyl-1 H -indol-3-yl)-5,7-dinitro-2,1,3-benzoxadiazole (6). 1,2-Dimethylindole (4) (60 mg, 0.41 mmol) was added to a solution of 4-chloro-5,7-dinitrobenzofurazan (2b) (100 mg, 0.41 mmol) in CHCl3 (4 ml), then the reaction mixture was maintained in darkness at room temperature for 1 day. The product was purified by column chromatography (eluent CHCl3). Yield 101 mg (70%), dark-blue crystals, mp 244-246°С. 1H NMR spectrum, δ, ppm (J, Hz): 2.53 (3Н, s, 2'-CН3); 3.93 (3Н, s, 1'-СН3); 7.12 (1Н, dd, J = 7.0, J = 7.7, Н-5'); 7.22-7.32 (2Н, m, H-4',6'); 7.56 (1Н, d, J = 8.0, Н-7'); 9.16 (1Н, s, Н-6). Found, m/z: 354.0832 [M+Н]+. C16H12N5O5. Calculated, m/z: 354.0833.
Synthesis of 4-(1,2-Dimethyl-1 H -indol-3-yl)-7-methyl-5,8а-dinitro-6,7,8,8а-tetrahydro-5а Н -[1, 2, 5]oxa-diazolo[3,4- е ]isoindole (10), 4-(1,2-Dimethyl-1 H -indol-3-yl)-7-methyl-5-nitro-7,8-dihydro-6 H -[1, 2, 5]oxadi-azolo[3,4- e ]isoindole (11), and 4-(1,2-Dimethyl-1 H -indol-3-yl)-7-methyl-5-nitro-7 H -[1,2,5]oxadiazolo[3,4- e ]-isoindole (12) (General Method). Compound 6 (100 mg, 0.28 mmol) was added to a suspension of finely ground sarcosine (7) (126 mg, 1.42 mmol) and paraform (51 mg, 1.70 mmol) in 4 ml of anhydrous benzene (for compound 10) or anhydrous MeCN (for compounds 11 and 12). The reaction mixture was refluxed until the color completely faded over 2 h, then the solution was cooled, filtered, the filtrate was evaporated, and the residue was purified by column chromatography (eluent CHCl3).
Compound 10. Mixture of diastereomers 10а and 10b in 3:2 ratio. Yield 108 mg (94%), brown crystals, mp 164-166°С. Found, m/z: 411.1406 [M+Н]+. C19H19N6O5. Calculated, m/z: 411.1411.
Diastereomer 10а. 1H NMR spectrum, δ, ppm (J, Hz): 2.32 (3Н, s, 2'-СН3); 2.44 (3Н, s, 7-СН3); 2.93 (1Н, dd, J = 7.6, J = 9.7) and 3.65 (1Н, dd, J = 8.5, J = 9.7, 6-СН2); 3.51 (1Н, d, J = 10.7) and 4.06 (1Н, d, J = 10.7, 8-СН2); 3.71 (3Н, s, 1'-CH3); 4.60 (1Н, dd, J = 7.6, J = 8.5, 5а-СН); 7.05-7.25 (3Н, m, Н-4',5',6'); 7.30 (1Н, d, J = 3.7, Н-7').
Diastereomer 10b. 1H NMR spectrum, δ, ppm (J, Hz): 2.33 (3Н, s, 2'-СН3); 2.42 (3Н, s, 7-СН3); 2.80 (1Н, dd, J = 7.5, J = 9.6) and 3.55 (1Н, dd, J = 8.4, J = 9.6, 6-СН2); 3.43 (1Н, d, J = 10.8) and 4.07 (1Н, d, J = 10.8, 8-СН2); 3.70 (3Н, s, 1'-CH3); 4.75 (1Н, dd, J = 7.5, J = 8.4, 5а-СН); 7.05-7.25 (3Н, m, Н-4',5',6'); 7.33 (1Н, d, J = 3.8, Н-7').
Compound 11. Yield 29 mg (29%), violet crystals, mp 208-210°С. 1H NMR spectrum, δ, ppm (J, Hz): 2.35 (3H, s, 2'-CH3); 2.69 (3Н, s, 7-CН3); 3.76 (3Н, s, 1'-СН3); 4.15 (1Н, ddd, J = 3.8, J = 3.8, J = 15.2) and 4.22 (1Н, ddd, J = 3.8, J = 3.8, J = 15.2, 8-СН2); 4.36 (1Н, ddd, J = 3.8, J = 3.8, J = 14.9) and 4.47 (1Н, ddd, J = 3.8, J = 3.8, J = 14.9, 6-СН2); 7.08 (1Н, dd, J = 6.8, J = 8.0, Н-5'); 7.13-7.25 (2Н, m, H-4',6'); 7.33 (1Н, d, J = 8.2, Н-7'). Found, m/z: 364.1404 [M+H]+. C19H18N5O3. Calculated, m/z: 364.1404.
Compound 12. Yield 5 mg (5%), brown crystals, mp 242-244°С. 1H NMR spectrum, δ, ppm (J, Hz): 2.35 (3Н, s, 2'-CН3); 3.74 (3H, s, 1'-CH3); 3.97 (3Н, s, 7-CН3); 7.06 (1Н, dd, J = 8.0, J = 7.9, Н-5'); 7.12-7.27 (3Н, m, H-8,4',6'); 7.31 (1Н, d, J = 8.1, H-7'); 7.64 (1Н, d, J = 2.0, Н-6). Found, m/z: 400.0811 [M+K]+. C19H15KN5O3. Calculated, m/z: 400.0806.
Synthesis of 4-(1-Benzyl-1 H -pyrrol-2-yl)-5,7-dinitro-2,1,3-benzoxadiazole (14) and 4-(1-Benzyl-1 H -pyrrol-3-yl)-5,7-dinitro-2,1,3-benzoxadiazole (15) (General Method). N-Benzylpyrrole (13) (128 mg, 0.82 mmol) was added to a solution of 4-chloro-5,7-dinitrobenzofurazan (2b) (100 mg, 0.41 mmol) in anhydrous MeCN (4 ml). The reaction mixture was maintained in darkness for 1 day, the solution was evaporated, the residue was purified by column chromatography (eluent 5:1 petroleum ether–EtOAc).
Compound 14. Yield 100 mg (67%), violet crystals, mp 120-122°С. 1H NMR spectrum, δ, ppm (J, Hz): 5.08 (2Н, s, CН2); 6.45 (1Н, dd, J = 2.7, J = 4.0, Н-4'); 6.73-6.83 (2Н, m, H-2,6 Ph); 6.85 (1Н, dd, J = 1.6, J = 4.0, H-3'); 7.08-7.15 (3Н, m, Н-3,4,5 Ph); 7.16 (1Н, dd, J = 1.6, J = 2.7, Н-5'); 8.78 (1Н, s, Н-6). Found, m/z: 366.0830 [M+H]+. C17H12N5O5. Calculated, m/z: 366.0833.
Compound 15. Yield 16 mg (11%), orange crystals, mp 148-150°С. 1H NMR spectrum, δ, ppm (J, Hz): 5.16 (2Н, s, CН2); 6.62 (1Н, dd, J = 1.9, J = 3.2, Н-4'); 6.79 (1Н, dd, J = 2.2, J = 3.2, Н-5'); 7.16-7.24 (2Н, m, H-2,6 Ph); 7.32-7.43 (3Н, m, Н-3,4,5 Ph); 7.98 (1Н, dd, J = 1.9, J = 2.2, Н-2'); 8.58 (1Н, s, Н-6). Found, m/z: 366.0832 [M+H]+. C17H12N5O5. Calculated, m/z: 366.0833.
Synthesis of 4-(1-Benzyl-1 H -pyrrol-2-yl)-7-methyl-5-nitro-7,8-dihydro-6 H -[1,2,5]oxadiazolo[3,4- e ]- isoindole (16) and 4-(1-Benzyl-1 H -pyrrol-2-yl)-7-methyl-5-nitro-7 H -[1,2,5]oxadiazolo[3,4- е ]isoindole (17) (General Method). Compound 14 (102 mg, 0.28 mmol) was added to a suspension of finely ground sarcosine (7) (126 mg, 1.42 mmol) and paraform (51 mg, 1.70 mmol) in anhydrous MeCN (7 ml). The reaction mixture was refluxed until the color completely faded over 1 h, then the solution was cooled, filtered, the filtrate was evaporated, the residue was purified by column chromatography (eluent 5:1 CHCl3–EtOAc), collecting the fractions with Rf = 0.9 (compound 17) and R f = 0.2 (compound 16).
Compound 16. Yield 30 mg (30%), orange crystals, mp 150-152°С. 1H NMR spectrum, δ, ppm (J, Hz): 2.65 (3H, s, 7-CH3); 4.06 (2Н, t, J = 3.7, 8-CH2); 4.36 (2Н, t, J = 3.7, 6-CH2); 5.08 (2Н, s, CH 2Ph); 6.32 (1Н, dd, J = 2.8, J =3.8, Н-4'); 6.46 (1Н, dd, J = 1.7, J = 3.8, Н-3'); 6.83-6.96 (3Н, m, Н-5', H-2,6 Ph); 7.12-7.24 (3Н, m, Н-3,4,5 Ph). Found, m/z: 398.1217 [M+Na]+. C20H17N5NaO3. Calculated, m/z: 398.1224.
Compound 17. Yield 3 mg (3%), brown crystals, mp 240-244°С. 1H NMR spectrum, δ, ppm (J, Hz): 3.94 (3Н, s, 7-CН3); 5.02 (2H, s, CH2); 6.26 (1Н, dd, J = 2.7, J = 3.6, Н-4'); 6.36 (1Н, dd, J = 1.8, J = 3.6, Н-3'); 6.80 (1Н, dd, J = 1.8, J = 2.7, Н-5'); 6.95-7.04 (2Н, m, H-2,6 Ph); 7.12-7.19 (4Н, m, H-8, Н-3,4,5 Ph); 7.58 (1Н, d, J = 2.0, Н-6). Found, m/z: 412.0803 [M+K]+. C20H15KN5O3. Calculated, m/z: 412.0806.
X-ray Structural Investigation of Compounds 6, 15, 16. Monocrystals of compound 6, obtained by crystallization from chloroform (C16H11N5O5, М 353.30) were monoclinic, space group P21/c, at 120 K: a 9.8327(8), b 7.0505(6), c 22.2720(18) Å; β 101.3423(14)°; V 1513.9(2) Å3; Z 4; d calc 1.550 g·cm-3; μ(MoKα) 1.19 cm-1; F(000) 728. The intensities of 17059 reflections were determined on a Bruker SMART APEX2 diffractometer (MoKα radiation, λ 0.71073 Å, ω-scanning, 2θ < 58°), 4037 independent reflections (R int 0.0211) were used in further refinement. The structure was solved directly and refined by least squares method in anisotropic full matrix approximation by F 2 hkl. The hydrogen atom positions were calculated geometrically and refined in isotropic approximation by the "rider" model. The final probability factor values for compound 6: wR 2 0.1152 and GOF 1.084 for all independent reflections (R 1 0.0445 calculated by F for 3508 observed reflections with I > 2σ(I)). All calculations were performed with the SHELXTL Plus software suite [25].
Monocrystals of compound 15 were obtained by crystallization from 1:5 mixture of EtOAc–petroleum ether (bp 40-70°С), C17H11N5O5, М 365.31, monoclinic, space group C2/c, at 120 K: a 30.905(2), b 4.7648(3), c 24.6937(16) Å; β 122.0190(10)°; V 3083.1(3) Å3; Z 8; d calc 1.574 g·cm-3; μ(MoKα) 1.20 cm-1; F(000) 1504. The intensities of 15007 reflections were determined on a Bruker SMART APEX2 diffractometer (MoKα radiation, λ 0.71073 Å, ω-scanning, 2θ < 58°), 4104 independent reflections (R int 0.0283) were used in the further refinement. The structure was solved directly and refined by least squares method in anisotropic full matrix approximation by F 2 hkl. The hydrogen atom positions were calculated geometrically and refined in isotropic approximation by the "rider" model. The final probability factor values for compound 15: wR 2 0.1018 and GOF 1.029 for all independent reflections (R 1 0.0383 calculated by F for 3402 observed reflections with I > 2σ(I)). All calculations were performed with the SHELXTL Plus software suite [25].
Monocrystals of compound 16 were obtained by crystallization from 1:5 mixture of EtOAc–petroleum ether (bp 40-70°С), (C20H17N5O3, М 375.39), monoclinic, space group P21/c, at 120 K: a 16.2477(10), b 8.9979(5), c 12.8559(8) Å; β 109.4650(10)°; V 1772.05(18) Å3; Z 4; d calc 1.407 g·cm-3; μ(MoKα) 0.908 cm-1; F(000) 784. Intensities of 20471 reflections were determined on a Bruker SMART APEX2 diffractometer (MoKα radiation, λ 0.71073 Å, ω-scanning, 2θ < 58°), 4716 independent reflections (R int 0.0324) were used in further refinement. The structure was solved directly and refined by least squares method in anisotropic full matrix approximation by F 2 hkl. The hydrogen atom positions were calculated geometrically and refined in isotropic approximation by the "rider" model. The final probability factor values for compound 16: wR 2 0.1156 and GOF 1.054 for all independent reflections (R 1 0.0450 calculated by F for 3763 observed reflections with I > 2σ(I)). All calculations were performed with the SHELXTL Plus software suite [25].
The X-ray structural analysis data for compounds 6, 15 and 16 were deposited at Cambridge Cystallographic Data Center (deposits CCDC 1029100, CCDC 1022755, and CCDC 1022778, respectively).
Ab initio quantum-chemical calculations were performed by DFT method in 6-31G** basis set, using the Gaussian 03 software suite [26] according to published methodology [27].
References
L. I. Hmelnitskii, S. S. Novikov, and T. I. Godovikova, Chemistry of Furoxans. Reactions and Applications [in Russian], Nauka, Moscow (1996), p. 430.
F. Terrier, in: Organic Reactivity: Physical and Biological Aspects, Royal Society of Chemistry, Cambridge (1995), p. 399.
F. Terrier, A. P. Chatrousse, Y. Soudais, and M. Hlaibi, J. Org. Chem., 49, 4176 (1984).
F. Terrier, M. J. Pouet, J. C. Halle, E. Kizilian, and E. Buncel, J. Phys. Org. Chem., 11, 707 (1998).
R. Goumont, E. Kizilian, E. Buncel, and F. Terrier, Org. Biomol. Chem., 1, 1741 (2003).
S. V. Kurbatov, Z. N. Budarina, G. S. Vaslyaeva, N. I. Borisenko, A. P. Knyazev, V. I. Minkin, Yu. A. Zhdanov, and L. P. Olekhnovich, Russ. Chem. Bull., Int. Ed., 46, 1445 (1997). [Izv. Akad. Nauk, Ser. Khim., 1509 (1997).]
V. A. Voronina, A. E. Frumkin, S. V. Kurbatov, A. M. Churakov, O. Yu. Smirnov, and L. P. Olekhnovich, Russ. Chem. Bull., Int. Ed., 51, 668 (2002). [Izv. Akad. Nauk, Ser. Khim., 617 (2002).]
P. G. Morozov, S. V. Kurbatov, F. M. Dolgushin, M. Yu. Antipin, and L. P. Olekhnovich, Russ. Chem. Bull., Int. Ed., 53, 2075 (2004). [Izv. Akad. Nauk, Ser. Khim., 1990 (2004).]
A. V. Tkachuk, S. V. Kurbatov, O. N. Burov, M. E. Kletskii, Yu. P. Tavunova, P. G. Morozov, V. A. Voronina, and V. I. Minkin, Russ. J. Org. Chem., 49, 1373 (2013). [Zh. Org. Khim., 49, 1388 (2013).]
V. I. Minkin, A. V. Tkachuk, M. E. Kletskii, D. V. Steglenko, V. A. Voronina, and S. V. Kurbatov, Russ. Chem. Bull., Int. Ed., 62, 464 (2013). [Izv. Akad. Nauk, Ser. Khim., 464 (2013).]
S. Kurbatov, P. Rodriguez-Dafonte, R. Goumont, and F. Terrier, Chem. Commun., 2150 (2003).
S. Kurbatov, A. Tatarov, V. Minkin, R. Goumont, and F. Terrier, Chem. Commun., 4279 (2006).
A. Tatarov, S. Kurbatov, G. Borodkin, R. Goumont, and F. Terrier, Tetrahedron, 66, 995 (2010).
F. Terrier, Modern Nucleophilic Aromatic Substitution, Wiley-VCH (2013), 488 p.
E. Buncel and F. Terrier, Org. Biomol. Chem., 8, 2285 (2010).
F. Terrier, J. M. Dust, and E. Buncel, Tetrahedron, 68, 1829 (2012).
S. Kurbatov, S. Lakhdar, R. Goumont, and F. Terrier, Org. Prep. Proced. Int., 44, 289 (2012).
S. A. Shevelev and A. M. Starosotnikov, Chem. Heterocycl. Compd., 49, 92 (2013). [Khim. Geterotsikl. Soedin., 102 (2013).]
C. Boga, E. Del Vecchio, L. Forlani, A. Mazzanti, and P. E. Todesco, Angew. Chem., Int. Ed., 44, 3285 (2005).
E. Pretsch, F. Bühlmann, and K. Affolter, Structure Determination of Organic Compounds [Russian translation], Mir, Moscow (2006), p. 438.
B. P. Nikolskii (editor), Chemist's Handbook [in Russian], Vol. 1, Khimiya, Moscow–Leningrad (1982), p. 352.
C. K. Prout, O. J. R. Hodder, and D. Viterbo, Acta Crystallogr., Sect. B.: Struct. Crystallogr. Cryst. Chem., B28, 1523 (1972).
H. Cerecetto and W. Porcal, Mini Rev. Med. Chem., 5, 57 (2005).
G. P. Sharnin, F. S. Levinson, S. A. Akimova, and R. Kh. Khasanov, USSR Inventor's Certificate 627129; Byul. Izobret., No. 37 (1978).
G. M. Sheldrick, SHELXTL, v. 5.10, Structure Determination Software Suite, Bruker AXS, Madison (1998).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, Gaussian 03, Revision D.01, Gaussian, Inc., Wallingford (2004).
D. V. Steglenko, M. E. Kletsky, S. V. Kurbatov, A. V. Tatarov, V. I. Minkin, R. Goumont, and F. Terrier, J. Phys. Org. Chem., 22, 298 (2009).
The quantum-chemical calculations and resonance spectroscopy studies of the structure and properties of nitrobenzofurazan derivatives were performed with support from the Russian Science Foundation (project No. 14-13-00103).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 12, pp. 1881-1891, December, 2014.
Rights and permissions
About this article
Cite this article
Semenyuk, Y.P., Kochubei, A.S., Morozov, P.G. et al. [3+2] Cycloaddition Reactions to Indolyl- and Pyrrolyl Derivatives of Dinitrobenzofurazan. Chem Heterocycl Comp 50, 1731–1740 (2015). https://doi.org/10.1007/s10593-015-1645-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1645-1