Abstract
Examining historical and contemporary processes underlying current patterns of genetic variation is key to reconstruct the evolutionary history of species and implement conservation measures promoting their long-term persistence. Combining phylogeographic and landscape genetic approaches can provide valuable insights, especially in regions harboring high levels of biodiversity that are currently threatened by climate and land cover changes, like southern Iberia. We used genetic (mtDNA and microsatellites) and spatial data (climate and land cover) to infer the evolutionary history and contemporary genetic connectivity in a short-range endemic salamander subspecies, Salamandra salamandra longirostris, using a combination of ecological niche modelling, phylogeographic, and landscape genetic analyses. Ecological-based analyses support a role of the Guadalquivir River Basin as a major vicariant agent in this taxon. The lower genetic diversity and greater differentiation of peripheral populations, together with analyses of climatically stable areas throughout time, suggest the persistence of a population in the central part of the current range since the Last Inter Glacial [LIG; ~ 120,000–140,000 years BP], and a micro refugium in the eastern end of the range. Habitat heterogeneity plays a major role in shaping patterns of genetic differentiation in S. s. longirostris, with forests representing key areas for its long-term persistence under scenarios of environmental change. Our study stresses the importance of maintaining population genetic connectivity in low-dispersal organisms under rapidly changing environments, and will inform management plans for the long-term survival of this evolutionarily distinct Mediterranean endemic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Identifying causative factors underlying patterns of genetic variation is a major goal in the fields of ecology, evolution, and conservation (Allendorf et al. 2013). Geographic and environmental heterogeneity are regarded as major drivers of gene flow and, by extension, of spatial patterns of genetic diversity and population divergence (Lee and Mitchell-Olds 2011; Wang et al. 2013; Sexton et al. 2014). Reduced gene flow (and high genetic divergence) among populations is expected when they are separated by increasing geographic distances (isolation by distance [IBD]; Wright 1943) and/or landscape and climatic barriers (i.e., landscape complexity is explicitly accounted for: isolation-by-resistance [IBR]; McRae 2006). Additionally, gene flow may also decrease due to ecological processes (e.g., natural and/or sexual selection against immigrants; reduced hybrid fitness) driven by environmental dissimilarity among sites, regardless of the geographic distance (or landscape complexity) separating them (isolation-by-environment [IBE]; Wang and Bradburd 2014).
Combining historical (i.e., phylogeography) and contemporary (i.e., landscape genetics) approaches provides a powerful framework for understanding the sequence of events and the relative role of geographic and ecological factors underlying genetic diversity (e.g., Zellmer and Knowles 2009; He et al. 2013; Velo-Antón et al. 2013; Rissler 2016; Noguerales et al. 2016; Zhang et al. 2016; Gutiérrez-Rodríguez et al. 2017a). These studies represent a window to the past and an opportunity to predict the fate of species, which is a conservation priority in regions with high levels of biodiversity that are also threatened by current climatic and landscape changes, like the southern Iberian Peninsula.
This region, located in one of the world´s biodiversity hotspots (Myers et al. 2000), is a geologically, climatically and topographically heterogeneous region. The interplay between these factors through geological time has promoted the persistence of genetically differentiated lineages in multiple isolated climatic refugia in response to Quaternary climatic oscillations. This resulted in high levels of endemicity and strong levels of population subdivision at the intra-specific level, documented for example across different species of amphibians and reptiles (Gonçalves et al. 2009; Velo-Antón et al. 2012a; Dias et al. 2015; Díaz-Rodríguez et al. 2015). However, rapid contemporary changes in habitat and climate in this region due to anthropogenic activities (mainly agricultural and deforestation activities) are threatening the long-term persistence of this biodiversity (Carvalho et al. 2011; Ferreira and Beja 2013). Amphibians are vulnerable to environmental changes because their ectothermic physiology is constrained by climate-related factors. Additionally, their relatively low dispersal ability (e.g., Smith and Green 2005) and dependence on humid environments and water bodies, which are essential to complete their life cycle, further exacerbate their vulnerability to changes in the surrounding environment (Stuart et al. 2004; Cushman 2006). Assessing the future viability of amphibian populations in this region requires understanding how extrinsic factors affect current patterns of genetic diversity and connectivity while accounting for their long and complex evolutionary history.
The fire salamander, Salamandra salamandra (Linnaeus 1758), is a terrestrial amphibian distributed throughout most of Europe. It occurs in humid and shaded environments with a wide range of vegetation communities, generally near ponds and streams where females deliver aquatic larvae (Velo-Antón et al. 2015). Much of the genetic and phenotypic intra-specific variation is found in the Iberian Peninsula (see Velo-Antón and Buckley 2015), with the southern tip of this region harboring a highly divergent lineage, S. s. longirostris. This lineage exhibits peculiar phenotypic traits, like a pointed snout, a characteristic pattern of dorsal coloration, and linear bone growth (Joger and Steinfartz 1994; Alcobendas and Castanet 2000; Donaire-Barroso et al. 2009). There is an ongoing debate about its taxonomic status (see Frost 2018) due to its geographic isolation, separated from other subspecies by the Guadalquivir River Basin (GRB), and deep divergence at the mtDNA level (~ 6% cyt-b divergence with S. s. crespoi and S. s morenica; García-París et al. 1998). However, its morphological, ecological and nuclear genetic differentiation have not been thoroughly studied to date. Divergence between S. s. longirostris and S. s. crespoi/morenica likely began with the formation of the Guadalquivir river valley during the Pliocene (García-París et al. 1998). Warm climate periods in the Quaternary, possibly associated with the existence of climatic refugia along the Betic range, could have also contributed to the diversification of southern Iberian S. salamandra lineages. The current allopatric and restricted distribution of S. s. longirostris in an area facing pronounced habitat loss has raised concern about its conservation, being catalogued as vulnerable to extinction (VU) according to the International Union for the Conservation of Nature (IUCN; Pleguezuelos 2004).
We used a combination of ecological niche modelling [ENM], phylogeographic, population and landscape genetic analyses to infer how historical and contemporary geographic and environmental heterogeneity have shaped current patterns of genetic variation in S. s. longirostris. Specifically, we used genetic (mtDNA and microsatellites) and spatial data (climate and land cover), covering the entire range of S. s. longirostris, to: (1) characterize patterns of genetic diversity and structure at historical (mtDNA) and contemporary (microsatellites) scales; (2) identify climatically stable areas that might have acted as climatic refugia during the Late Pleistocene; and (3) investigate how environmental and geographic factors (climate, habitat and topography) have driven genetic divergence at deeper (phylogeographic) and shallower (landscape genetic) evolutionary scales.
Materials and methods
Sampling and laboratory procedures
Sampling was conducted in areas with potentially suitable habitat for S. s. longirostris (i.e., humid areas in woodlands and mountains, and rocky areas with abundant vegetation and water bodies) across its entire range (south of the GRB; Fig. 1). A total of 156 tissue samples (tail or toe clips), obtained mostly from larvae, were collected and georeferenced at 26 localities (Online Resource 2.1).
Distribution range of S. s. longirostris (in green) and neighbouring S. salamandra populations (in red) separated by the Gualdalquivir River Basin. Cells denote 10 × 10 km distribution records of S. salamandra based on herpetological atlases from Spain. Inset represents the distribution of S. salamandra with the allopatric distribution of S. s. longirostris in the southern Iberian Peninsula. Black circles indicate sampling locations for molecular analyses. (Color figure online)
DNA was extracted from tissue using the EasySpin® Genomic DNA Tissue Kit (Citomed, Lisbon, Portugal), following the manufacturer’s protocol. Quality of extracted DNA was checked by electrophoresis on 0.8% agarose gels. Extracted DNA was used as template in polymerase chain reactions (PCR) to amplify: (I) one mitochondrial fragment, the mitochondrially encoded cytochrome b and adjacent tRNAs (hereafter cyt-b; ca. 1400 bp); and (II) nine microsatellite loci (Sal29, Sal23, SalE7, SalE5, SalE2, SalE06, Sal3, SalE8, SalE12; Steinfartz et al. 2004).
The cyt-b fragment was amplified for 42 samples covering the entire range of S. s. longirostris, using the primers Glu14100L (forward, 5′ GAA AAA CCA AYG TTG TAT TCA ACT ATA A 3′) and Pro15500H (reverse, 5′ AGA ATT YTG GCT TTG GGT GCCA 3′) (Zhang et al. 2008) following the protocol described in Beukema et al. (2016). Sequences were edited and aligned by eye in GENEIOUS version 11.1.4 (http://www.geneious.com/). One individual of S. s. crespoi and another one representing S. s. morenica were also sequenced for cyt-b to calculate genetic distances between subspecies (Online Resource 2.1). The nine microsatellites were amplified for most samples (n = 146). They were distributed in four optimized multiplex reactions (panels S2, S3, S4 and S5; see also Online Resource 2.2) and amplified following the conditions described in Álvarez et al. (2015). Quality and quantity of PCR products for cyt-b and microsatellites were verified on 2% agarose gels. Cycle sequencing reactions for cyt-b were carried out using the ABI Prism® BigDye® Terminator version 3.1 Cycle Sequencing Kit (Applied Biosystems, Carlsbad, CA, USA) standard protocol. An ABI Prism® 3130xl Genetic Analyser Sequencer (Applied Biosystems/HITASHI) was then used to sequence the cyt-b fragments for both strands and to genotype microsatellites. Allele scoring was performed using GeneMapper version 4.0 (Applied Biosystems/HITASHI).
Phylogenetic analyses
We used the software BEAST version 1.8.4 (Drummond et al. 2012) to perform coalescent-based Bayesian phylogenetic inference on S. s. longirostris cyt-b sequences. We selected the optimal nucleotide substitution model (TrN) with JMODELTEST version 2.1.4 (Darriba et al. 2012), under the Bayesian information criterion (BIC). For BEAST analyses, we ran analyses using both a lognormal relaxed clock and a strict clock, with a constant population size model as the coalescent tree prior. Three independent runs for each clock model were performed and combined with a total of 50 million generations (burn-in: 10%). Tree files of all runs were combined using the software LOGCOMBINER version 1.7.5, and parameter convergence was verified by examining the effective sample sizes (ESSs) in TRACER version 1.6. A maximum clade credibility summary tree with Bayesian posterior probabilities (BPP) for each node was obtained using TREEANNOTATOR version 1.8.4. The resulting tree was visualized and edited with FigTree version 1.4.3 (http://tree.bio.ed.ac.uk/software/figtree). BEAST analyses were run in the Cipres Science Gateway (Miller et al. 2010). Genetic distances (uncorrected p-distances) were calculated between subspecies and between clades within S. s. longirostris using GENEIOUS.
Microsatellite pre-treatment procedures
The microsatellite data set was subjected to several procedures to reduce potential statistical biases. First, genotyped samples presenting more than 20% of missing data were discarded. Second, locations with a sample size ≥ 10 were considered local random mating units (i.e. demes or populations). Following these criteria, a total of seven populations/demes were considered (MSI, PLAG, LMO, AVI, VLR, TOR and VVR; Table 1; Online Resource 2.1) for subsequent population and landscape genetic analyses. Because of low sample sizes in some locations (n < 10; Online Resource 2.1), we drew 2-km radius buffers (roughly the maximum dispersal distance recorded in Salamandra salamandra; Hendrix et al. 2017) around each sample in ArcGIS version 10.1 (ESRI) and pooled samples if their buffers intersected. Third, sampling individuals in early life stages (e.g., amphibian larvae) may introduce significant biases in routine microsatellite quality control tests and genetic diversity estimates (e.g., Hardy–Weinberg equilibrium [HWE] and Linkage Equilibrium [LE]; Sánchez-Montes et al. 2017), as well as in landscape genetics analyses (Peterman et al. 2016; Wang 2018). This is because larvae are often spatially clustered, increasing the likelihood of sampling relatives and consequently, inflating inter-population genetic divergence. To address this shortcoming, we screened familial relationships independently for each of the seven populations using COLONY 2.0.6.1 (Jones and Wang 2010). Analyses were performed using the full-likelihood method, with high likelihood precision and medium run length, assuming polygamy for both sexes. No a priori information regarding known parents was provided. The remaining parameters were kept as default. If a pair of related individuals (parent-offspring, half- or full-siblings) had a posterior probability higher than 0.50, then one of them was removed from the dataset. After excluding related individuals, we re-estimated population genetic diversity and differentiation parameters to assess the effect of including relatives in results. Related individuals were discarded from downstream genetic analyses when estimates including (full dataset) and excluding relatives correlated below r = 0.9. Finally, the presence of null alleles was assessed using a maximum likelihood estimator implemented in INEst version 2.0 (Chybicki and Burczyk 2009). Analyses for each of the seven demes were run under the individual inbreeding model, with a total of 200,000 cycles thinned every 50 cycles and a burn-in of 10%. GENEPOP version 4.5.1 (Rousset 2008) was used to test for deviations from HWE and LE (10,000 dememorization steps, 1000 batches and a batch length of 10,000 iterations). The p-value (p < 0.05) of the HWE and LE multiple exact tests was corrected using the false discovery rate (Benjamini and Hochberg 1995).
Contemporary genetic diversity and structure
To characterize genetic diversity in the seven demes, observed heterozygosity (HO), expected heterozygosity (HE), mean number of alleles (NA) and mean population relatedness (R; Queller and Goodnight 1989) were estimated in GenAlEx version 6.502 (Peakall and Smouse 2012). Additionally, unbiased allelic richness (AR) and private allelic richness (P-AR) were calculated in HP-RARE version 1.0 (Kalinowski 2005). Two measures of population genetic differentiation were estimated and compared: (1) pairwise FST (Weir and Cockerham 1984), calculated in R (R Development Core Team 2015) package diveRsity version 1.9.89 (Keenan et al. 2013); and (2) the conditional genetic distance (cGD) (Dyer and Nason 2004), estimated in R package gstudio version 1.3 (Dyer 2014). Respective 95% confidence intervals (CIs) were calculated based on 1000 bootstrap replicates, and pairwise estimates were considered significant when 95% CIs did not overlap zero.
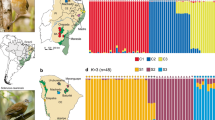
Population genetic structure was assessed using the Bayesian algorithm implemented in STRUCTURE version 2.3.4 (Pritchard et al. 2000). Ten independent runs were performed for a number of clusters (K) ranging between 1 and 8. A burn-in period of 100,000 iterations, followed by 1,000,000 iterations, with a correlated allele frequencies admixture model and no prior information regarding population of origin was set for each run. The best K describing the observed genetic data was identified with STRUCTURE HARVESTER version 0.6.94 (Earl and vonHoldt 2012), following two independent criteria: (1) the K exhibiting the highest mean value of likelihood (Pritchard et al. 2000); and (2) the K showing the lowest ΔK statistic value (Evanno et al. 2005). For the optimal K, runs were summarized and graphically displayed using Pophelper version 1.0.10 (Francis 2017).
Additionally, a discriminant analysis of principal components (DAPC; Jombart et al. 2010), a multivariate method implemented in R package adegenet version 2.0.1 (Jombart et al. 2008), was used for an independent description of population structure, because this method does not assume HWE or LE. DAPC summarizes the data to minimize genetic differentiation within groups while maximizing it between groups, potentially unravelling complex structures, like hierarchical clustering or clinal differentiation (e.g., isolation by distance; Jombart et al. 2010). A prior number of genetic clusters of K = 7 (the lowest value obtained in the analysis comparing clustering solutions using the Bayesian Information Criterion, BIC) was chosen, while the number of retained principal components and discriminant functions were selected following the package’s manual guidelines to avoid overfitting.
Ecological niche-based modelling
Ecological niche-based models (ENMs) were used to reconstruct present and past (Last Interglacial [LIG; ~ 120,000–140,000 years BP], Last Glacial Maximum [LGM; ~ 21,000 years BP] and Mid-Holocene [~ 6000 years BP]) climatic niches of S. s. longirostris with the maximum entropy algorithm implemented in MAXENT version 3.3.3 k (Phillips et al. 2006). MAXENT has shown to be highly reliable when using presence-only data and small datasets (Elith et al. 2010).
ENMs for the present were built using 28 occurrence records obtained from our own fieldwork, collaborators, and from the Spanish Herpetological Atlas (Online Resource 2.3). To avoid statistical biases arising from spatial autocorrelation (Fourcade et al. 2014), a maximum of one presence record per 10 × 10 km grid cell and a minimum distance of 2 km (maximum distance recorded for the species; Hendrix et al. 2017) was allowed. The Nearest Neighbour index function of ArcGIS (ESRI 2012) was used to assess the degree of data clustering (z-score = − 0.435). From the 19 climatic variables with a 1 km2 resolution downloaded from WorldClim (Hijmans et al. 2005, http://www.worlcim.org), we retained six climate variables (with pairwise Pearson’s r ≤ 0.75) to represent contemporary climatic conditions and avoid the possible statistical effects of collinearity among predictor variables on niche modeling: Isothermality, Mean Temperature of Wettest Quarter, Mean Temperature of Driest Quarter, Mean Temperature of Coldest Quarter, Annual Precipitation and Precipitation of Warmest Quarter. Models built with current conditions were projected to past climates (LIG, LGM, Mid-Holocene) using the same six variables. LGM variables were downloaded at 2.5 arc minutes resolution (~ 5 × 5 km), while the remaining variables were downloaded at 30 arc-seconds resolution (~ 1 × 1 km).
The distribution area for model construction, validation and projections was created using a 200-km radius buffer around occurrence records. Bootstrap subsampling was used to build 50 model replicates, retaining 30% of the data records in order to test each replicate. The “Fade by clamping” option was used for past projections to avoid spurious model projections (Elith et al. 2010). Default values were used for all other settings. Model performance was evaluated using the area under the receiver operating characteristic curve (AUC) in MAXENT, which ranges from 0.5 (complete randomness) to 1 (perfect discrimination) (Phillips et al. 2006).
Consensus models and projections to past climatic conditions were converted to binary projections (i.e., presence/absence) using the minimum training presence threshold (suitability values higher or equal to the lowest value given to a distribution record are considered suitable areas). The four obtained suitability maps (Current, Mid-Holocene, LGM (CCSM and MIROC) and LIG) were then overlapped in ArcGIS to identify stable climatic areas over time (i.e., common potential areas of occurrence which could serve as refugia in all time periods).
Landscape genetic analyses
We assessed the effect of contemporary geographic heterogeneity on gene flow by calculating matrices of pairwise resistance distances in CIRCUITSCAPE version 4.0 (McRae 2006) to test for patterns of IBD and IBR. CIRCUITSCAPE calculates averaged pairwise resistances to gene flow among populations based on all possible paths (unlike the least-cost path), thus better explaining the movement of genes among widely separated regions over many generations (McRae et al. 2008). For the IBD model, a matrix of geographic distances was estimated from a raster layer depicting a “flat” landscape (i.e. a value of one in all cells). Patterns of IBR were examined through the calculation of three independent matrices of pairwise resistance distances (IBRCLIMATIC, IBRSLOPE and IBRNDVI). We first used MAXENT to generate independent ENMs based on: (1) the six bioclimatic variables; (2) slope; and (3) the Normalized Difference Vegetation Index (NDVI; 250 × 250 m). Then, each ENM map was used as input for the program CIRCUITSCAPE to to generate pairwise resistance matrices for each model (Online Resources 1.1 and 2.4).
Prior to matrix calculation of the IBRCLIMATIC, IBRSLOPE and IBRNDVI models, we converted suitability scores (S) of each ENM layer’s cell (S ranges from 0, unsuitable, to 1, suitable) to resistance scores (R) using a simple arithmetic operation (R = (1 − S)/100). In all CIRCUITSCAPE analyses, the pairwise mode and a cell connection scheme of eight neighbours were set, while remaining parameters were kept as default.
The role of environmental dissimilarity (IBE) on genetic differentiation was assessed by estimating climate (IBECLIMATIC) and NDVI (productivity index; IBENDVI) dissimilarity matrices between deme locations. Local environmental variation of the six bioclimatic variables was captured by a principal component analysis, with “varimax” rotation applied to the values of the six climatic variables extracted from the location of populations, occurrence records and 1000 randomly generated points in the study area. Then, the three principal components of each population location were selected and climatic dissimilarities were obtained by calculating pairwise Euclidean distances among populations. Principal component analyses were performed using IBM SPSS version 24.0 (IBM Corp., Armonk, NY, USA). Habitat dissimilarities were obtained by calculating Euclidean distances between population location values extracted from the previously built NDVI-based ENM model.
Matrices of geographic (IBD), resistance (IBRCLIMATIC, IBRNDVI and IBRSLOPE) and environmental (IBECLIMATIC and IBENDVI) heterogeneity were regressed against matrices of pairwise genetic differentiation (pairwise FST and cGD). Univariate regressions with randomizations were used, which is a suitable approach when the hypothesis to be tested strictly concerns distances (Legendre and Fortin 2010). Furthermore, to quantify the independent effects of each factor on genetic differentiation, we implemented a multivariate matrix regression with randomization approach (MMRR, Wang 2013). We first examined multicollinearity among our predictor variables using Pearson´s correlation tests and variance inflation factors (VIF) (Zuur et al. 2010; Prunier et al. 2015).
Results
Phylogenetic analyses
The cyt-b dataset comprised 979 base pairs (17 variable and 11 parsimony informative), and we recovered 19 haplotypes among the 42 samples analyzed. An identical phylogenetic tree topology was obtained in analyses assuming a lognormal or a strict molecular clock. In both cases, we recovered two well-supported clades (BPP > 0.95) and a third moderately supported clade (BPP = 0.9) (Fig. 2). One main clade groups most of the samples (n = 31) and covers the central range of S. s. longirostris, with a sister clade (n = 5) including samples at the southern end of the range. The third clade (n = 6) is sister to the other two clades and includes the easternmost populations. Salamandra s. longirostris sequences differed by 6% and 7% from S. s. crespoi and S. s. morenica, respectively, while S. s. crespoi and S. s. morenica differed 2%. Intra-clade distances within S. s. longirostris were low (1%).
Map depicting climatically stable areas, suitable for S. s. longirostris, obtained through overlapping past (LIG, LGM and mid-Holocene) and present ENMs. A Bayesian phylogenetic tree based on cyt-b is also shown, with colors representing the three mtDNA clades discussed in the text. Black and grey dots represent nodes with posterior probability higher than 0.95 or equal to 0.9, respectively. The scale indicates the proportion of substitutions/site along the branch.
Microsatellite pre-treatment procedures
A total of 146 samples were successfully genotyped. Nine individuals were identified as full-siblings, but including them did not significantly affect genetic diversity and population differentiation estimates (r > 0.99 in the full vs. reduced dataset). Therefore, all samples were retained for downstream genetic analyses (Online Resource 2.1).
No microsatellite loci showed evidence of null alleles. Three pairs of loci showed signs of linkage disequilibrium, though deviations of LE were not consistent among populations. Additionally, significant deviations from HWE were only found in the easternmost population (VVR) due to the presence of four monomorphic loci. Since deviation from LE and HWE were not consistent across populations, we used all loci for subsequent analyses.
Contemporary genetic diversity and genetic structure
The easternmost population (VVR) showed extremely low genetic diversity (NA = 2.22, HO = 0.31, HE = 0.27, and AR = 2.01) and high relatedness values (R = 0.82) in comparison with the central group including the other six populations (Table 1). Within this group, the easternmost population (TOR) had the lowest genetic diversity values, followed by the westernmost population (MSI) (Table 1).
Population genetic differentiation was high overall (mean FST = 0.24; mean cGD = 2.69), with VVR exhibiting the highest genetic differentiation (range FST = 0.38–0.56; range cGD = 4.10–5.19), followed by TOR (range FST = 0.22–0.30; range cGD = 1.93–3.51) (Table 2). STRUCTURE identified K = 2 as the number of clusters best describing genetic structure (based on both the Ln Pr(X/K) and the ∆K methods), separating the easternmost population VVR from the remaining populations (Fig. 3). The second most supported K (K = 5) showed high levels of admixture among central populations in the main group (MSI, PLAG, LMO, AVI), with low or no admixture at the range margins (Fig. 3). DAPC results were congruent with STRUCTURE’s output, with the highest differentiation in the easternmost population (VVR), followed by the two easternmost populations from the central group (TOR and VLR) (Online Resource 3.1).
Ecological niche-based modelling
The ENM had high AUC values (AUC = 0.977), suggesting good predictive power. Among variables used in the model, Annual precipitation and Mean temperature of the driest quarter presented the highest contribution values (Online Resource 3.2). Climatically suitable areas identified for the present (Online Resource 3.3) largely depict the known current distribution of S. s. longirostris, with the exception of some areas north of the GRB. Climatically suitable areas identified for past scenarios (LIG, LGM and mid-Holocene) varied considerably, with LIG showing a suitable area similar to that for the present interglacial (Online Resource 3.3), while projections to the mid-Holocene and especially the LGM predicted larger suitable areas (Online Resource 3.3). Stable climatic areas derived from the intersection of these projections identified a continuous area concomitant with the current distribution range of S. s. longirostris as the main potential climatic refuge. In addition, small and partially disconnected areas were identified at the eastern end of the range and north of the GRB (Fig. 2).
Landscape connectivity and landscape genetic analyses
Habitat and climatic-based ENMs had moderate to high AUC values, respectively (Online Resource 2.4). The connectivity analysis performed with CIRCUITSCAPE revealed overall moderate or high connectivity among populations of S. s. longirostris for all studied variables (climate, habitat, topography and distance), with the exception of population VVR, which is isolated by an area of ca. 50 Km from its closest population (Fig. 4).
Univariate regressions between ecological (IBD, IBRCLIMATIC, IBRNDVI, IBRSLOPE, IBECLIMATIC and IBENDVI) and genetic matrices (FST and cGD) revealed significant (p < 0.05) positive relationships, with the exception of both IBE matrices (Table 3). Univariate regressions using IBRNDVI provided the highest coefficients of determination (r2 = 0.96 and r2 = 0.88 for FST and cGD, respectively; Table 3). Moreover, MMRR analyses retrieved an overall significant (p < 0.05) coefficient of determination of 0.99 and 0.92 for FST and cGD, respectively, though IBRNDVI was the only significant (p < 0.05) predictor when regressed against pairwise FST (Table 4). Multicollinearity, however, might have influenced these models, as the input variables were not independent, showing pairwise correlation values and VIF values above the traditionally considered thresholds (i.e. r > 0.7 and VIF > 10), especially in IBRCLIMATIC and IBRNDVI (Online Resource 2.5).
Discussion
Contemporary patterns of genetic diversity and structure are the result of complex evolutionary processes occurring at different temporal depths (e.g., Velo-Antón et al. 2013; Zhang et al. 2016; Gutiérrez-Rodríguez et al. 2017a). Our results highlight the importance of both historical and current factors in shaping patterns of genetic variation in a Mediterranean relict, S. s. longirostris, with implications for the conservation of this taxon.
The Guadalquivir River Basin as a vicariant agent in S. s. longirostris
Previous phylogenetic studies have shown deep mtDNA divergence in S. s. longirostris with respect to other population lineages in S. salamandra (e.g., García-París et al. 1998, 2003; Steinfartz et al. 2000; Vences et al. 2014; Beukema et al. 2016). This, coupled with their disjunct distribution, led some authors to postulate GRB as the major vicariant agent promoting isolation and subsequent genetic differentiation of S. s. longirostris south of the GRB, a pattern in common with other co-distributed species (e.g., Santos et al. 2012; Velo-Antón et al. 2012a). Our palaeoclimatic niche modeling analyses have revealed the potential occurrence of areas suitable for S. s. longirostris north and south of the GRB since the LIG. Thus, it is possible that areas north of the GRB might have been occupied by S. s. longirostris during the LGM, which later became extinct because of environmental changes and/or competition with other species, including other lineages of S. salamandra. Alternatively, areas north of the GRB may have never been colonized by S. s. longirostris due to the physical barrier imposed by the GRB, which likely favored an allopatric differentiation process from neighbouring lineages of Salamandra (S. s. crespoi/S. s. morenica). These scenarios can be further tested through the assessment of historical patterns of nuclear gene flow between S. s. longirostris and these lineages, which may have also been affected by the physical barrier imposed by the GRB.
Distribution dynamics and historical and contemporary patterns of genetic variation
Patterns of genetic structure at both historical (mtDNA) and contemporary scales (microsatellites) were mostly concordant, and support the predictions based on ENMs, with peripheral populations (VVR, TOR, VLR and MSI) exhibiting lower genetic diversity and greater genetic differentiation when compared to populations near the core of the distribution (Fig. 3; Tables 1, 2). This pattern has also been reported for eastern European populations of S. salamandra at similar geographic scales (Najbar et al. 2015; Konowalik et al. 2016).
Climatic oscillations during the Pleistocene promoted cycles of population expansion and contraction, which likely caused demographic and genetic effects on peripheral versus central populations of S. s. longirostris. During range expansions, peripheral populations at the expansion front are subjected to serial founder events, with small effective population sizes resulting in spatial patterns of decreasing genetic diversity and increasing genetic divergence through processes like drift or allele surfing (e.g., Excoffier et al. 2009; Pereira et al. 2018). The high genetic diversity observed in central populations is consistent with demographic stability associated with the existence of a climatic refugium in this area. In contrast, peripheral populations of S. s. longirostris are located in areas where climatic suitability varied through time, which probably promoted recurrent population extinctions followed by recolonization (founder) events by individuals from central populations, resulting in the low levels of genetic diversity and high genetic differentiation observed (Excoffier et al. 2009). Among peripheral populations, the easternmost population (VVR) shows divergent mtDNA haplotypes (1%; Fig. 2), although one haplotype is shared with one individual in the geographically closest population (TOR; ~ 100 km distant). The level of genetic divergence between S. s. longirostris clades (1%) suggests their separation occurred during the Pleistocene. The population VVR also showed extremely low levels of genetic diversity (HO = 0.3) and high differentiation (FST > 0.5). This pattern reflects the genetic effects of long-term isolation and demographic instability, which have also been assessed in other populations of S. salamandra, including insular populations isolated during the Holocene (Velo-Antón et al. 2012b), or small-sized urban populations (Lourenço et al. 2017).
In addition to the homogeneous, climatically stable area inferred by ENMs in the core of the range, from the southernmost coast (strait of Gibraltar) to the Grazalema mountains at the Cádiz and Málaga border, an isolated climatically stable area was inferred to occur at the easternmost end of the range of S. s. longirostris (northwest of the Tejeda and Almijara mountains in Málaga), which is presently occupied by VVR. Thus, climatic fluctuations have probably had a major effect on historical patterns of population connectivity in S. s. longirostris by alternatively promoting isolation and recolonization dynamics through changes in habitat suitability.
Environmental factors shaping current genetic structure in S. s. longirostris
Models characterizing geographical heterogeneity (i.e., IBD and IBR models) were better predictors of patterns of genetic differentiation in S. s. longirostris than IBE models. This suggests that gene flow between populations in different ecological settings is not limited by selection against dispersers or by individual preferences to remain in a particular environment (Wang 2013; Wang and Bradburd 2014). Moreover, the IBRNDVI showed the strongest statistical association with genetic structure. This result highlights the greater importance of landscape composition and structure (in this case the presence of forested or agricultural areas, Online Resource 3.4) on genetic connectivity relative to climate, stressing the crucial role of forests in maintaining gene flow among salamander populations (e.g., Cushman 2006; Todd et al. 2009). The importance of preserving forest habitats is particularly pressing for southern Iberian populations of S. salamandra. Forests are extremely important for salamander survival, providing food resources, shelters, and aquatic habitats for reproduction (Egea-Serrano et al. 2006; Romero et al. 2013). The importance of forests and vegetation acting as microclimate buffers in this area and in neighboring regions has also been emphasized for other amphibian species (e.g., Dias et al. 2015; Escoriza and Hassine 2014).
Overall, our landscape genetics approach suggests that models incorporating both geographic distances and ecologically relevant climate or landscape features provide better predictions of observed genetic patterns at fine spatial scales (e.g., Muñoz-Pajares et al. 2017; Gutiérrez-Rodríguez et al. 2017b). Unfortunately, it was not possible to disentangle the relative role of each variable in our statistical framework. This is a recurring problem in landscape genetic studies arising from the non-independence of predictor variables (i.e., collinearity) in regression analyses, which inflates the variance of regression parameters and ultimately leads to the spurious identification of relevant predictors in the model (Dormann et al. 2013).
Implications for conservation under rapid changing environments
Salamandra s. longirostris is a good example of the ongoing biodiversity crisis faced by Iberian amphibians due to the rapid climatic (Carvalho et al. 2011) and landscape changes (Ferreira and Beja 2013). It also represents the high levels of endemicity found in southern Iberia, and the evolutionary distinctiveness of allopatric lineages with restricted ranges occurring in this area (e.g., Santos et al. 2012; Velo-Antón et al. 2012a; Dias et al. 2015), many of which have low vagility, which makes them particularly vulnerable to rapid environmental changes. Maintaining population genetic connectivity is key to preserve evolutionary potential and, consequently, to ensure their long-term persistence (Baguette et al. 2013). Reduced gene flow can result in lower effective population sizes and genetic diversity, causing genetic erosion and eventually local extinction events (Frankham 2005; Cushman 2006). Our results reveal limited genetic connectivity (and low genetic diversity) in peripheral populations (mainly the easternmost population). It is thus crucial to preserve forested patches between central and peripheral populations, but especially among the latter, to maintain population connectivity and help to ensure the long-term conservation of S. s. longirostris. Such forested habitats also play a crucial role as microrefugia, which may reduce the risk of local extinction of fire salamanders and other species under predicted scenarios of climate change in the Iberian Peninsula (Carvalho et al. 2011). These results have general implications for biodiversity conservation under rapidly changing environments (e.g., Velo-Antón et al. 2013; Noguerales et al. 2016; Carvalho et al. 2017; Razgour et al. 2017) but can also inform management plans for the long term survival of this short-range endemic salamander. Two management units corresponding to the central and eastern population groups can be established, with the latter requiring urgent action to compensate the negative effects of reduced genetic variation on long-term population persistence.
References
Alcobendas M, Castanet J (2000) Bone growth plasticity among populations of Salamandra salamandra: interactions between internal and external factors. Herpetologica 56:14–26
Allendorf FW, Luikart GH, Aitken SN (2013) Conservation and the genetics of populations, 2nd edn. Wiley, Chichester
Álvarez D, Lourenço A, Oro D, Velo-Antón G (2015) Assessment of census (N) and effective population size (Ne) reveals consistency of Ne single-sample estimators and a high Ne/N ratio in an urban and isolated population of fire salamanders. Conserv Genet Resour 7:705–712
Baguette M, Blanchet S, Legrand D, Stevens VM, Turlure C (2013) Individual dispersal, landscape connectivity and ecological networks. Biol Rev 88:310–326
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57:289–300
Beukema W, Nicieza AG, Lourenço A, Velo-Antón G (2016) Colour polymorphism in Salamandra salamandra (Amphibia: Urodela), revealed by a lack of genetic and environmental differentiation between distinct phenotypes. J Zool Syst Evol Res 54:127–136
Carvalho SB, Brito JC, Crespo EG, Watts ME, Possingham HP (2011) Conservation planning under climate change: toward accounting for uncertainty in predicted species distributions to increase confidence in conservation investments in space and time. Biol Conserv 144:2020–2030
Carvalho SB, Velo-Antón G, Tarroso P, Portela AP, Barata M, Carranza S, Mortiz C, Possingham HP (2017) Spatial conservation prioritization of biodiversity spanning the evolutionary continuum. Nat Ecol Evol 1:0151
Chybicki IJ, Burczyk J (2009) Simultaneous estimation of null alleles and inbreeding coefficients. J Hered 100:106–113
Cushman SA (2006) Effects of habitat loss and fragmentation on amphibians: a review and prospectus. Biol Conserv 128:231–240
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772–772
Dias G, Beltrán JF, Tejedo M, Benítez M, González-Miras E, Ferrand N, Gonçalves H (2015) Limited gene flow and high genetic diversity in the threatened Betic midwife toad (Alytes dickhilleni): evolutionary and conservation implications. Conserv Genet 16:459–476
Díaz-Rodríguez J, Gonçalves H, Sequeira F, Sousa-Neves T, Tejedo M, Ferrand N, Martínez-Solano I (2015) Molecular evidence for cryptic candidate species in Iberian Pelodytes (Anura, Pelodytidae). Mol Phylogenet Evol 83:224–241
Donaire-Barroso D, González de la Vega JP, Barnestein JAM (2009) Aportación sobre los patrones de diseño pigmentario en Salamandra longirostris Joger and Steinfartz, 1994, y nueva nomenclatura taxonómica. Butll Soc Cat Herp 18:10–17
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz JRG, Gruber B, Lafourcade B, Leitão PJ, Münkemüller T (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:27–46
Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973
Dyer RJ (2014) Gstudio: a Package for the spatial analysis of population genetic marker data. Virginia Commonwealth University, Richmond
Dyer RJ, Nason JD (2004) Population graphs: the graph- theoretic shape of genetic structure. Mol Ecol 13:1713–1728
Earl DA, vonHoldt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361
Egea-Serrano A, Oliva-Paterna FJ, Torralva M (2006) Breeding habitat selection of Salamandra salamandra (Linnaeus, 1758) in the most arid zone of its European distribution range: application to conservation management. Hydrobiologia 560:363–371
Elith J, Kearney M, Phillips S (2010) The art of modelling range-shifting species. Methods Ecol Evol 1:330–342
Escoriza D, Hassine JB (2014) Microclimatic variation in multiple Salamandra algira populations along an altitudinal gradient: phenology and reproductive strategies. Acta Herpetol 9:33–41
ESRI (2012) ArcGIS 10.1. ESRI, Redlands
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Excoffier L, Foll M, Petit RJ (2009) Genetic consequences of range expansions. Annu Rev Ecol Evol Syst 40:481–501
Ferreira M, Beja P (2013) Mediterranean amphibians and the loss of temporary ponds: Are there alternative breeding habitats? Biol Conserv 165:179–186
Fourcade Y, Engler JO, Rödder D, Secondi J (2014) Mapping species distributions with MAXENT using a geographically biased sample of presence data: a performance assessment of methods for correcting sampling bias. PLoS ONE 9:e97122
Francis RM (2017) Pophelper: an R package and web app to analyse and visualize population structure. Mol Ecol Resour 17:27–32
Frankham R (2005) Genetics and extinction. Biol Conserv 126:131–140
Frost DR (2018) Amphibian species of the world. Online resource. http://research.amnh.org/herpetology/amphibia/index.html
García-París M, Alcobendas M, Alberch P (1998) Influence of the Guadalquivir River Basin on mitochondrial DNA evolution of Salamandra salamandra (Caudata: Salamandridae) from southern Spain. Copeia 1998:173–176
García-París M, Alcobendas M, Buckley D, Wake DB (2003) Dispersal of viviparity across contact zones in Iberian populations of fire salamanders (Salamandra) inferred from discordance of genetic and morphological traits. Evolution 57:129–143
Gonçalves H, Martínez-Solano I, Pereira RJ, Carvalho B, García-París M, Ferrand N (2009) High levels of population subdivision in a morphologically conserved Mediterranean toad (Alytes cisternasii) result from recent, multiple refugia: evidence from mtDNA, microsatellites and nuclear genealogies. Mol Ecol 18:5143–5160
Gutiérrez-Rodríguez J, Barbosa AM, Martínez-Solano I (2017a) Present and past climatic effects on the current distribution and genetic diversity of the Iberian Spadefoot toad (Pelobates cultripes): an integrative approach. J Biogeogr 44:245–258
Gutiérrez-Rodríguez J, Goncalves J, Civantos E, Martínez-Solano I (2017b) Comparative landscape genetics of pond-breeding amphibians in Mediterranean temporal wetlands: the positive role of structural heterogeneity in promoting gene flow. Mol Ecol 26:5407–5420
He Q, Edwards DL, Knowles LL (2013) Integrative testing of how environments from the past to the present shape genetic structure across landscapes. Evolution 67:3386–3402
Hendrix R, Schmidt BR, Schaub M, Krause ET, Steinfartz S (2017) Differentiation of movement behavior in an adaptively diverging salamander population. Mol Ecol 26:6400–6413
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Joger U, Steinfartz S (1994) Zur subspezifischen Gliederung der siidiberischen Feuersalamander (Salamandra salamandra-Komplex). Abh Ber Nat 17:83–98
Jombart T, Devillard S, Dufour A-B, Pontier D (2008) Revealing cryptic spatial patterns in genetic variability by a new multivariate method. Heredity 101:92–103
Jombart T, Devillard S, Balloux F (2010) Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genet 11:94
Jones OR, Wang J (2010) COLONY: a program for parentage and sibship inference from multilocus genotype data. Mol Ecol Resour 10:551–555
Kalinowski ST (2005) HP-RARE 1.0: a computer program for performing rarefaction on measures of allelic richness. Mol Ecol Resour 5:187–189
Keenan K, McGinnity P, Cross TF, Crozier WW, Prodöhl PA (2013) diveRsity: an R package for the estimation and exploration of population genetics parameters and their associated errors. Methods Ecol Evol 4:782–788
Konowalik A, Najbar A, Babik W, Steinfartz S, Ogielska M (2016) Genetic structure of the fire salamander Salamandra salamandra in the Polish Sudetes. Amphib Reptil 37:405–415
Lee C-R, Mitchell-Olds T (2011) Quantifying effects of environmental and geographical factors on patterns of genetic differentiation. Mol Ecol 20:4631–4642
Legendre P, Fortin MJ (2010) Comparison of the Mantel test and alternative approaches for detecting complex multivariate relationships in the spatial analysis of genetic data. Mol Ecol Resour 10:831–844
Lourenço A, Álvarez D, Wang IJ, Velo-Antón G (2017) Trapped within the city: integrating demography, time since isolation and population-specific traits to assess the genetic effects of urbanization. Mol Ecol 26:1498–1514
Martínez-Freiría F, Velo-Antón G, Brito JC (2015) Trapped by climate: interglacial refuge and recent population expansion in the endemic Iberian adder Vipera seoanei. Divers Distrib 21:331–344
McRae BH (2006) Isolation by resistance. Evolution 60:1551–1561
McRae BH, Dickson BG, Keitt TH, Shah VB (2008) Using circuit theory to model connectivity in ecology, evolution, and conservation. Ecology 89:2712–2724
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Gateway Computing Environments Workshop (GCE), 2010, pp 1–8
Muñoz-Pajares AJ, García C, Abdelaziz M, Bosch J, Perfectti F, Gómez JM (2017) Drivers of genetic differentiation in a generalist insect-pollinated herb across spatial scales. Mol Ecol 26:1576–1585
Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Najbar A, Babik W, Najbar B, Ogielska M (2015) Genetic structure and differentiation of the fire salamander Salamandra salamandra at the northern margin of its range in the Carpathians. Amphib Reptil 36:301–311
Noguerales V, García-Navas V, Cordero PJ, Ortego J (2016) The role of environment and core-margin effects on range-wide phenotypic variation in a montane grasshopper. J Evol Biol 29:2129–2142
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics 28:2537–2539
Pereira P, Teixeira J, Velo-Antón G (2018) Allele surfing shaped the genetic structure of the European pond turtle via the colonization and population expansion across the Iberian Peninsula from Africa. J Biogeogr. https://doi.org/10.1111/jbi.13412
Peterman W, Brocato ER, Semlitsch RD, Eggert LS (2016) Reducing bias in population and landscape genetic inferences: the effects of sampling related individuals and multiple life stages. PeerJ 4:e1813. https://doi.org/10.7717/peerj.1813
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Model 190:231–259
Pleguezuelos JM (2004) Atlas y libro rojo de los anfibios y reptiles de España. Organismo Autónomo de Parques Nacionales, Madrid
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Prunier JG, Colyn M, Legendre X, Nimon KF, Flamand MC (2015) Multicollinearity in spatial genetics: separating the wheat from the chaff using commonality analyses. Mol Ecol 24:263–283
Queller DC, Goodnight KF (1989) Estimating relatedness using genetic markers. Evolution 43:258–275
R Development Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Razgour O, Taggart JB, Manel S, Juste J, Ibáñez C, Rebelo H, Alberdi A, Gareth J, Park K (2017) An integrated framework to identify wildlife populations under threat from climate change. Mol Ecol Resour. https://doi.org/10.1111/1755-0998.12694
Rissler LJ (2016) Union of phylogeography and landscape genetics. Proc Natl Acad Sci USA 113:8079–8086
Romero D, Olivero J, Real R (2013) Comparative assessment of different methods for using land-cover variables for distribution modelling of Salamandra salamandra longirotris. Environ Conserv 40:48–59
Rousset F (2008) genepop’007: a complete re-implementation of the genepop software for Windows and Linux. Mol Ecol Resour 8:103–106
Sánchez-Montes G, Ariño AH, Vizmanos JL, Wang J, Martínez-Solano I (2017) Effects of sample size and full sibs on genetic diversity characterization: a case study of three syntopic Iberian pond-breeding amphibians. J Hered 108:535–543
Santos X, Rato C, Carranza S, Carretero MA, Pleguezuelos JM (2012) Complex phylogeography in the Southern Smooth Snake (Coronella girondica) supported by mtDNA sequences. J Zool Syst Evol Res 50:210–219
Sexton JP, Hangartner SB, Hoffmann AA (2014) Genetic isolation by environment or distance: which pattern of gene flow is most common? Evolution 68:1–15
Smith MA, Green DM (2005) Dispersal and the metapopulation paradigm in amphibian ecology and conservation: are all amphibian populations metapopulations? Ecography 28:110–128
Steinfartz S, Veith M, Tautz D (2000) Mitochondrial sequence analysis of Salamandra taxa suggests old splits of major lineages and postglacial recolonizations of Central Europe from distinct source populations of Salamandra salamandra. Mol Ecol 9:397–410
Steinfartz S, Kuesters D, Tautz D (2004) Isolation and characterization of polymorphic tetranucleotide microsatellite loci in the Fire salamander Salamandra salamandra (Amphibia: Caudata). Mol Ecol Resour 4:626–628
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues AS, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306:1783–1786
Todd BD, Luhring TM, Rothermel BB, Gibbons JW (2009) Effects of forest removal on amphibian migrations: implications for habitat and landscape connectivity. J Appl Ecol 46:554–561
Van Oosterhout C, Hutchinson WF, Wills DP, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Resour 4:535–538
Velo-Antón G, Buckley D (2015) Salamandra común–Salamandra salamandra (Linnaeus 1758). In: Enciclopedia virtual de los vertebrados españoles. Museo Nacional de Ciencias Naturales (MNCN), CSIC, Madrid
Velo-Antón G, Godinho R, Harris DJ, Santos X, Martínez-Freiria F, Fahd S, Larbes S, Pleguezuelos JM, Brito JC (2012a) Deep evolutionary lineages in a Western Mediterranean snake (Vipera latastei/monticola group) and high genetic structuring in Southern Iberian populations. Mol Phylogenet Evol 65:965–973
Velo-Antón G, Zamudio KR, Cordero-Rivera A (2012b) Genetic drift and rapid evolution of viviparity in insular fire salamanders (Salamandra salamandra). Heredity 108:410–418
Velo-Antón G, Parra JL, Parra-Olea G, Zamudio KR (2013) Tracking climate change in a dispersal-limited species: reduced spatial and genetic connectivity in a montane salamander. Mol Ecol 22:3261–3278
Velo-Antón G, Santos X, Sanmartín-Villar I, Cordero-Rivera A, Buckley D (2015) Intraspecific variation in clutch size and maternal investment in pueriparous and larviparous Salamandra salamandra females. Evol Ecol 29:185–204
Vences M, Sanchez E, Hauswaldt JS, Eikelmann D, Rodríguez A, Carranza S, Donaire D, Gehara M, Helfer V, Lötters S, Werner P, Schulz S, Steinfartz S (2014) Nuclear and mitochondrial multilocus phylogeny and survey of alkaloid content in true salamanders of the genus Salamandra (Salamandridae). Mol Phylogenet Evol 73:208–216
Wang IJ (2013) Examining the full effects of landscape heterogeneity on spatial genetic variation: a multiple matrix regression approach for quantifying geographic and ecological isolation. Evolution 67:3403–3411
Wang J (2018) Effects of sampling close relatives on some elementary population genetics analyses. Mol Ecol Resour 18:41–54
Wang IJ, Bradburd GS (2014) Isolation by environment. Mol Ecol 23:5649–5662
Wang IJ, Glor RE, Losos JB (2013) Quantifying the roles of ecology and geography in spatial genetic divergence. Ecol Lett 16:175–182
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Wright S (1943) Isolation by distance. Genetics 28:114–138
Zellmer AJ, Knowles LL (2009) Disentangling the effects of historic vs. contemporary landscape structure on population genetic divergence. Mol Ecol 18:3593–3602
Zhang P, Papenfuss TJ, Wake MH, Qu L, Wake DB (2008) Phylogeny and biogeography of the family Salamandridae (Amphibia: Caudata) inferred from complete mitochondrial genomes. Mol Phylogenet Evol 49:586–597
Zhang YH, Wang IJ, Comes HP, Peng H, Qiu YX (2016) Contributions of historical and contemporary geographic and environmental factors to phylogeographic structure in a Tertiary relict species, Emmenopterys henryi (Rubiaceae). Sci Rep 6:24041
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14
Acknowledgements
We thank David Buckley, David Donaire, Francisco Jiménez Cazalla, Jesús Díaz-Rodríguez, Luis García-Cardenete and Saúl Yubero for providing samples and help with field work. S. Lopes helped with genotyping. Fieldwork for obtaining tissue samples was done with the corresponding permits from the regional administrations This work was funded by FEDER funds through the Operational Programme for Competitiveness Factors—COMPETE—and by National Funds through FCT—Foundation for Science and Technology—under the PTDC/BIA-EVF/3036/2012, PTDC/BIA-BEC/099915/2008, POCI-01-0145-FEDER-006821 and FCOMP- 01-0124-FEDER-028325. GVA, HG, GCD, AL and PT are supported by FCT (IF/01425/2014, SFRH/BPD/102966/2014, SFRH/BD/89750/2012, PD/BD/106060/2015, SFRH/BPD/93473/2013), respectively.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Antunes, B., Lourenço, A., Caeiro-Dias, G. et al. Combining phylogeography and landscape genetics to infer the evolutionary history of a short-range Mediterranean relict, Salamandra salamandra longirostris. Conserv Genet 19, 1411–1424 (2018). https://doi.org/10.1007/s10592-018-1110-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-018-1110-7