Abstract
Genetic pollution through introgressive hybridization of local species by exotic relatives is a major, yet neglected aspect of biological invasions, particularly in amphibians where human introductions are frequent. In Western Switzerland, crested newts make an interesting case: the Italian species Triturus carnifex was introduced at least a century ago within the range of the native and threatened T. cristatus. To understand the genetic consequences of this introduction and inform wildlife management authorities, we conducted a genetic survey on the remaining northern crested newt populations known in the area, using newly-developed species-diagnostic nuclear (microsatellites) and mitochondrial (control region) DNA markers. We documented massive nuclear introgression by the T. carnifex genome, which has completely replaced T. cristatus in most populations, especially in the Geneva area where the introduction was originally reported. However, many of these individuals retained the ancestral T. cristatus mtDNA, which could be explained by asymmetric introgression between the two species, stemming from demographic and/or selective processes. Analyses of genetic diversity support multiple events of T. carnifex releases, most-likely of proximate North Italian origin. We pinpointed the last indigenous populations in the region and recommend to prioritize their protection. Our study demonstrates the invasive potential of introduced taxa through introgressive hybridization, alerts about the underestimated rate of illegal amphibian translocations, and emphasizes the need for genetic analyses to monitor such invasions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The artificial introduction of non-native species raises serious concerns for the conservation of local biodiversity (Allendorf et al. 2001; Laikre et al. 2010). These threats may be ecological, i.e. interspecific competition with local taxa, alteration of trophic chains, and transmission of exotic diseases. In parallel, threats may also be genetic, as introduced individuals can potentially hybridize with local relatives and compromise their genetic integrity through introgression (Allendorf et al. 2001). This process is a sensitive issue for wildlife managers, the status of introgressed populations being usually controversial (Allendorf et al. 2001). Moreover, the consequences of introgression may greatly differ depending on the genetic and environmental factors involved. Introgressive hybridization with a distant relative may lower the fitness of individuals (i.e. outbreeding depression; as both species can be, at least partly, reproductively isolated), potentially precipitating the decline of local populations (e.g. Dufresnes et al. 2015b). Alternatively, ecological and genetic factors may interact to promote the invasion of exotic genes, and thus the rapid replacement of the native genome (Fitzpatrick et al. 2010). These effects may be exacerbated in the case of multiple introductions and when local populations are small, which is typically the case in most threatened species (Laikre et al. 2010).
Well-documented cases of massive genetic introgression raising important conservation issues mostly comes from mammals (e.g. red deers by sikas, Senn et al. 2010; minks by polecats, Cabria et al. 2011; wolves by domestic dogs, Godinho et al. 2011; Randi and Lucchini 2002; Randi et al. 2014; wild by domestic cats, Oliveira et al. 2015; between marten species, Kyle et al. 2003), and to some extent birds (Barilani et al. 2007) and fish (in particular due to large-scale commercial releases of non-native salmonids, Laikre et al. 2010). The matter has received less attention in amphibians, where intentional or accidental human translocations are frequent (Smith and Sutherland 2014), thus raising many opportunities for genetic pollution in native populations (Johnson et al. 2010; Austin et al. 2011; Dufresnes et al. 2015b; Meilink et al. 2015). Given the unpredictability of this process, case-by-case surveys using genetic tools are essential to understand the genetic consequences of human introductions and inform managing authorities (Fitzpatrick et al. 2010; Laikre et al. 2010).
The northern crested newt Triturus cristatus is one of the most threatened, widespread amphibians in Europe, and owing to population declines it is red-listed in the majority of countries/regions where it occurs (Edgar and Bird 2006; Dufresnes and Perrin 2015). It is known to naturally come into contact with its southern relative, the Italian crested newt T. carnifex, across a narrow hybrid zone in central Europe (Maletzky et al. 2008, 2010), whereas both species are geographically isolated by the Alpine arc in the rest of their ranges (Wielstra et al. 2013, cf. Fig 1). However, human introductions of T. carnifex have been documented throughout the 20th century in several Western-European countries, and cases of hybridization with local T. cristatus were reported in the United Kingdom (Brede et al. 2000), Southern-Germany (Franzen et al. 2002), and the Netherlands (Meilink et al. 2015).
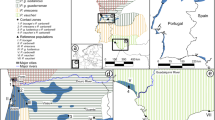
Bayesian clustering of microsatellite genotypes with STRUCTURE (nuclear) and mitotypes of crested newts from Western Switzerland (green: T. cristatus, red: T. carnifex). The map displays the average STRUCTURE assignments of each population. Natural distributions of the two species, known other introductions of T. carnifex (red crosses), location of the study area (red rectangle) and the reference populations are shown on the top-left frame. Photo credits: Radek Sejkora (T. carnifex) and Andréas Meyer (T. cristatus)
In Switzerland, T. cristatus occurs north of the Alps across the Swiss Plateau (cf. Fig 1), but populations have been dramatically collapsing over the past decades (Schmidt and Zumbach 2005). The species is now listed as endangered by national authorities (Schmidt and Zumbach 2005). As for most Swiss amphibians, habitat degradation and loss are mainly responsible for this sharp decline, even more so for T. cristatus which requires large and deep sunny ponds with rich vegetation and is very sensitive to pollutants (Meyer et al. 2009). Moreover, in Western Switzerland, T. carnifex was presumably introduced multiple times in the area of Geneva, at least since the beginning of the 20th century (Arntzen and Thorpe 1999; Arntzen 2001), raising additional conservation concerns. Most probably, the species was originally imported for zoological experiments and released at the University of Geneva (Wittenberg 2005); in this part of range, the Alps form an impassable dispersal barriers and both species always had strongly disruptive natural distributions (Fig. 1; Triturus newts are not found above 1100 m in Switzerland, Grossenbacher 1988; Meyer et al. 2009). Genetic analyses based on allozyme polymorphism hence indicated that Geneva crested newts clustered with T. carnifex, suggesting that native T. cristatus had already been replaced in this area (Arntzen and Thorpe 1999; Arntzen 2001). Population monitoring based on morphological criteria also suggested that T. cristatus morphotypes had disappeared from the Geneva area and that hybrid forms now predominate (Ferrantin 2007). However, distinguishing between these two species in the field is difficult, but more so because hybrids show intermediate characters (Brede et al. 2000); therefore, monitoring this introduction requires genetic tools to infer species assignments and the degree of introgression.
In order to understand the genetic consequences of this introduction and its extent in the region, we conducted a comprehensive genetic survey of the last known crested newt populations in Western Switzerland. Based on cross-amplifying and newly-developed fast-evolving nuclear and mitochondrial markers, we characterized their taxonomic assignment and extent of admixture, which will be important to inform future management strategies.
Methods
Sampling and DNA extraction
Crested newts were captured from 29 localities (n = 203 individuals) scattered across the Lake Geneva region, from the Geneva area to the upper Rhone Valley, which correspond to the remaining stations where it is known to occur. To characterize their nature and origin, reference populations of T. cristatus (n = 97 individuals from 11 localities) and T. carnifex (n = 33 individuals from 3 localities) were also sampled from different parts of their natural ranges. Details on sample origins are available in Additional File S1. DNA was obtained from buccal swabs (study area; Broquet et al. 2007) or tail tips (preserved in absolute ethanol; reference populations), and extracted using the Qiagen Biosprint Robotic workstation.
Microsatellite genotyping and mtDNA sequencing
To infer their nuclear background, individuals were genotyped for 11 microsatellite loci, cross-amplifying in both species and featuring interspecific polymorphism. Five loci were optimized from Krupa et al. (2002) and six loci were developed for this study. The latter were isolated from a library of T. carnifex DNA enriched with microsatellite repeat motifs (GIS, Chatsworth, CA), tested and chosen for cross-amplification in T. cristatus, and for species-specific polymorphisms. Markers and primer sequences are listed in Table 1.
Markers were amplified separately in 10µL PCRs with 2µL of DNA template (10–100 ng), 1 × Qiagen PCR buffer, 0.5 mM of each primer, 0.25 mM of dNTPs, 0.25 units of Qiagen Taq, and either 0.19 mM (for Tcri13, Tcri35, Tcri29, Tcri46), 0.33 mM (for Tcri36) or 0.21 mM (for A7, A126, D1, D5, A8, D127) of MgCl2. PCRs were run as follows: (a) for Tcri13, Tcri35, Tcri29, Tcri46, Tcri36: 2′ at 94 °C, 39× (1′ at 94 °C, 1′ at annealing temperature, 1′ at 72 °C), and 5′ at 72 °C; (b) for A7, A126, D1, D5, A8, D127: 2′ at 94 °C, 35× (30″ at 94 °C, 30″ at annealing temperature, 1′ at 72 °C) and 5′ at 72 °C. PCR products were pooled for genotyping as followed. (a) Mix 1:Tcri13 (diluted 4.5×), Tcri35 (diluted 1.8×) and Tcri46 (diluted 4.5×) with respective ratios of 1:2.5:1; (b) Mix 2: Tcri29 (diluted 7×) and Tcri36 ((diluted 7×) with a ratio 1:1; (c) Mix 3: A7 and A126 with a ratio of 1:1; (d) Mix 4: D1 and D5 with a ratio of 1:1; (e) Mix 5: A8 (diluted 2×) and D127 (diluted 4×) with ratio 1:1. Pooled amplicons were then run on an ABI Prism 3100 genetic analyzer; peaks were scored with GeneMapper 4.0 (Applied Biosystem).
To infer the mitochondrial background of Western–Swiss crested newts, we analyzed 644 bp of the mitochondrial control region (CR) in a total of 100 individuals (91 from the study area, plus 9 from reference populations). Two adjacent parts of this marker were amplified by two separate PCRs using newly developed primers conserved across urodeles: L-Uro/H- Uro (375–376 bp amplicon) and L-CR-Uro/H-tRNAPhe-Uro (385 bp amplicon). Primer sequences are available in Table 1. Both amplicons were amplified in 25µL PCRs with 2µL of DNA template (10–100 ng), 1 × Qiagen PCR buffer, 0.15 mM of MgCl2, 0.25 mM of dNTPs, 0.5 mM of each primer, and 0.625 units of Qiagen Taq. PCRs were run as follows: 3′ at 95 °C, 35 × (30″ at 95 °C, 30″ at 55 °C, 30″ at 72 °C) and 5′ at 72 °C. Amplicons were purified using the Promega purification kit and sequenced in both directions on an ABI3730 genetic analyzer (Applied Biosystems). Quality-checked sequences obtained from each amplicon were concatenated and aligned for subsequent analyses.
Population genetic analyses
For reference populations with large sample sizes (n ≥ 9; T. cristatus: loc. 1–3, 10–11; T. carnifex: loc. 41–42), we checked for the presence of genotyping errors and null alleles with Microchecker (Oosterhout et al. 2004), and used Arlequin (Excoffier et al. 2005) to calculate Hardy–Weinberg Equilibrium (HWE) and linkage disequilibrium between loci.
Several analyses were conducted to characterize the genetic structure of Western–Swiss crested newts. First, we performed Bayesian clustering assignment of individual microsatellite genotypes using STRUCTURE (Pritchard et al. 2000). We tested from 1 to 11 groups (K), with 10 replicate runs per K, each run consisting of 100,000 iterations (after a burnin’ of 10,000). The ΔK test was applied to determine the most likely number of groups (Evanno et al. 2005) using STRUCTURE HARVESTER (Earl and VonHoldt 2012). Replicates of the most likely solution were combined using CLUMPP (Jakobsson and Rosenberg 2007) and graphical displays of individual ancestry coefficients (barplots) were built using DISTRUCT (Rosenberg 2003). Second, we conducted a principal component analysis on microsatellite genotypes (adegenet R package, Jombart 2008).
Relationships between mitochondrial haplotypes were reconstructed by a maximum-likelihood phylogenetic inference with PhyML (Guindon and Gascuel 2003), using a HKY model of sequence evolution (MrAIC, Nylander 2004) and 1,000 bootstrap replicates. A published sequence of T. marmoratus (Wielstra and Arntzen 2011, GenBank HQ697279) was used as outgroup.
Results
Data quality
Potential null alleles were detected in a few instances for A126 (loc. 1, 11 and 42), D1 (loc. 1) and A8 (loc. 3). Accordingly, A126 and D1 were the only markers significantly departing from HWE in some T. cristatus reference populations (A126: loc. 1 and 11; D1: loc.1) after Bonferroni corrections. We did not detect significant linkage disequilibrium for any pair of loci in any reference population.
Clustering of microsatellite genotypes
Bayesian clustering assignment using STRUCTURE unambiguously recovered two groups (ΔK = 2452.1; Additional File S2), corresponding to the gene pools of T. cristatus and T. carnifex, based on our reference populations (Fig. 1). In Western Switzerland, all individuals from the Geneva area (loc. 12–31) were assigned by nuclear loci to T. carnifex, with evidence for admixture in only three individuals (i.e. with ancestry coefficients below 0.9; e.g. loc. 21). Populations distributed further east along the lake coast displayed a mixed pattern: some are deeply introgressed by T. carnifex (loc. 32, 35–37, 38), while others retained most (loc. 33–34) if not all the local T. cristatus nuclear genome (loc. 39–40).
The Principal Component Analysis (PCA) on microsatellite genotypes confirmed this result: the first axis discriminated between the two species (Additional File S3), while the second contrasted southern (loc. 42–43) versus northern T. carnifex native populations (loc. 41). Most Western–Swiss newts clearly group with the latter, and only a few populations remained similar to T. cristatus. Some individuals had intermediate scores on the first axis, indicative of genetic admixture.
Distribution of mitochondrial lineages
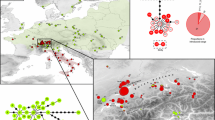
Phylogenetic reconstruction of CR haplotypes allowed perfect discrimination between the two species (3 % of divergence, Fig. 2; GenBank KU743871-KU743879). Reference T. cristatus (n = 6 sequences) displayed three different haplotypes (CRI1-3), with only one haplotype (CRI1) occurring in Western Switzerland (out of n = 33 sequences). Four T. carnifex haplotypes (CAR1-3, CAR6) were recovered in our study area (out of n = 44 sequences), with the most abundant (CAR1) also present in Northern Italy (loc. 41). In contrast, reference individuals from Southern Italy (loc. 42–43) possessed specific haplotypes, not found elsewhere (CAR4-5).
Interestingly, distributions of the two mtDNA lineages across our study area contrast with the nuclear pattern: 40 % of the newts sampled on the west end of the lake retained T. cristatus mtDNA despite being assigned by microsatellites to T. carnifex (25 out of 58 sequenced individuals; Fig. 2). Furthermore, admixed (loc. 33–34, 38) and pure (based on microsatellites, loc. 39–40) T. cristatus populations only host the native T. cristatus mtDNA.
Discussion
Our genetic survey revealed that the introduction of exotic T. carnifex in the Geneva area has been followed by massive introgressive hybridization and genetic replacement of the native crested newt T. cristatus across Western Switzerland. This pattern parallels the situation in Dutch crested newt populations, where genetic pollution of local T. cristatus by introduced T. carnifex resulted in the formation of an artificial, geographically continuous hybrid zone (Meilink et al. 2015).
Two lines of evidence support replacement by introgression rather than ecological exclusion only. First, several newts harbored direct signs of nuclear admixture, suggesting incomplete backcrossing (loc. 12, 21, 32–34 and 38). Second, cyto-nuclear discordance was detected in as many as 25 specimens. Interestingly, the discordance was strongly asymmetric: about half of nuclearly pure T. carnifex retained the mtDNA of T. cristatus but not the reverse, i.e. we found no pure (or admixed) T. cristatus carrying T. carnifex mtDNA. Given that mtDNA is only maternally-transmitted, this pattern may reflect male-biased dispersal of T. carnifex, coupled with allele surfing in expanding populations (Klopfstein et al. 2006). It has been shown that during the first phases of expansions, introgression can be an asymmetric process going from the local to the invading species, irrespective of relative densities, with local alleles surfing at the edge of the expansion wave, thus reaching high frequencies in the invading species. This pattern is predicted to be stronger for organelle markers with low effective size such as mtDNA (Currat et al. 2008; Petit and Excoffier 2009).
Alternatively, or in addition, this pattern could stem from reproductive isolation between the two species, for instance due to asymmetric incompatibilities between reciprocal hybrid crosses, i.e. lower fitness of offspring from ♂ T. cristatus × ♀ T.carnifex crosses compared to ♂ T. carnifex × ♀ T.cristatus crosses. So far, laboratory experiments did not find evidence for differential performance between T. cristatus and T. cristatus × T. carnifex hybrid larvae (Wyssmüller 2007). Yet, in the Netherlands, hybrids between invasive T. carnifex and local T. cristatus may face negative selection across their artificial hybrid zone (i.e. the transition appears bimodal, suggesting selection against hybrids, Meilink et al. 2015). Asymmetric viability was detected directly in hybrid crosses between T. cristatus and another European relative, the marbled newt T. marmoratus (Arntzen et al. 2009), although it is more distantly related than T. carnifex (Wielstra and Arntzen 2011). Here, T. cristatus and T. carnifex diverged as early as ~9 Mya (Wielstra and Arntzen 2011) and have thus likely accumulated reproductive isolation. In amphibians, complete isolation is often observed after shorter divergences in the wild (Pleiocene, <5 Mya; Dufresnes et al. 2015a), and is likely maintained by pre-zygotic barriers (e.g. assortative mating). Accordingly, T. carnifex and T. cristatus present sharp transitions and hardly hybridize across their natural contact zone in Eastern Europe (Maletzky et al. 2008). In introduced ranges, as observed in Swiss and Dutch crested newts, hybridization could occur more frequently due to the lack of opportunity for assortative mate choice (i.e. the introduced species is initially present at low density). Even distantly-related species may thus pose a threat to the genetic integrity of native taxa (Dufresnes et al. 2015b).
Our data indicate a probable North Italian origin of T. carnifex in Western Switzerland, as previously suggested (Arntzen 2001). Microsatellites clearly distinguished between the southern and northern lineages of T. carnifex (Canestrelli et al. 2012), and all Swiss genotypes unambiguously clustered with the latter (Additional File S3). The low number of available mitochondrial sequences from native T. carnifex population (n = 3, only 1 from North-Italian individuals), however, prevents to confirm this origin. Yet, the presence of four different T. carnifex mtDNA haplotypes in the Geneva area suggests multiple releases of exotic specimens. Historical records suspected at least one introduction of T. carnifex in Geneva, possibly close to population 26 (Grossenbacher et al. 2012 and ref. therein). Furthermore, unlike for the Dutch introduction (Meilink et al. 2015), here the expansion of T. carnifex eastward along Lake Geneva’s shore (loc. 32, 35–37, 38) was discontinuous and more likely resulted from additional illegal translocations rather than natural dispersal. Two arguments support this hypothesis. First, in Western Switzerland, crested newts greatly suffer from the lack of suitable breeding sites and the last remaining populations are disconnected, strongly limiting natural dispersal (Fig. 1). Second, individuals have been presumably translocated in the past decades in several additional places (Geneva area and loc. 35–37, 38; S. Dubey and JT pers. com.), where the T. carnifex genome is now present.
Our results provide insights on the future management of these populations. First, frequent monitoring should inform on the progress of invasive T. carnifex across Western Switzerland and adjacent regions. Second, conservation efforts should be prioritized on the last indigenous T. cristatus populations (loc. 39–40, but also loc. 33–34 which are relatively free of introgression), both to protect their breeding sites and prevent future introductions. Furthermore, if hybrids face asymmetric incompatibilities, massive regular translocations of female T. cristatus may help restoring the native gene pool. Exhaustive experimental studies, involving all types of possible crosses and at different stages (larval and adults of F1 s and F2 s), are obviously required to characterize potential incompatibilities beforehand. Future surveys should also focus on the ecology of T. carnifex in its introduced range, in comparison to T. cristatus, in order to better understand the ecological causes and consequences of the invasion (e.g. competitive exclusion). In contrast to T. cristatus, introduced T. carnifex is associated to anthropogenically-disturbed ponds in Western Switzerland, which may partly explain its success in this heavily-impacted region (Ferrantin 2007).
The case of crested newts in Western Switzerland emphasizes the invasive potential of introduced taxa through genetic introgression (“genetic pollution”). This aspect of invasions is often neglected (Laikre et al. 2010), particularly in amphibians where morphological similarities between closely-related species and their hybrids complicate population monitoring (e.g. Dubey et al. 2014, this study). Rates of illegal amphibian translocations may be underestimated (Fisher and Garner 2007; Smith and Sutherland 2014), and our study contributes to understanding the extent and genetic consequences of anthropogenic introductions on indigenous related taxa.
References
Allendorf FW, Leary RF, Spruell P, Wenburg JK (2001) The problems with hybrids: setting conservation guidelines. Trends Ecol Evol 16:613–622
Arntzen JW (2001) Genetic variation in the Italian crested newt, Triturus carnifex, and the origin of a non-native population north of the Alps. Biodivers Conserv 10:971–987
Arntzen JW, Thorpe RS (1999) Italian crested newts (Triturus carnifex) in the basin of Geneva: distribution and genetic interactions with autochthonous species. Herpetologica 55:423–433
Austin JD, Gorman TA, Bishop D, Moler P (2011) Genetic evidence of contemporary hybridization in one of North America’s rarest anurans, the Florida bog frog. Anim Conserv 14:553–561
Barilani M, Sfougaris A, Giannakopoulos A et al (2007) Detecting introgressive hybridisation in rock partridge populations (Alectoris graeca) in Greece through Bayesian admixture analyses of multilocus genotypes. Conserv Genet 8:343–354
Brede E, Thorpe RS, Arntzen JW, Langton TES (2000) A morphometric study of a hybrid newt population (Triturus cristatus/T. carnifex): Beam Brook Nurseries, Surrey, U.K. Biol J Linn Soc 70:685–695
Broquet T, Berset-Braendli L, Emaresi G, Fumagalli L (2007) Buccal swabs allow efficient and reliable microsatellite genotyping in amphibians. Conserv Genet 8:509–511
Cabria MT, Michaux JR, Gómez-Moliner BJ et al (2011) Bayesian analysis of hybridization and introgression between the endangered european mink (Mustela lutreola) and the polecat (Mustela putorius). Mol Ecol 20:1176–1190
Canestrelli D, Salvi D, Maura M et al (2012) One species, three pleistocene evolutionary histories: phylogeography of the Italian crested newt, Triturus carnifex. PLoS One 7:e41754
Currat M, Ruedi M, Petit RJ, Excoffier L (2008) The hidden side of invasions: massive introgression by local genes. Evolution 62:1908–1920
Dubey S, Leuenberger J, Perrin N (2014) Multiple origins of invasive and “native” water frogs (Pelophylax spp.) in Switzerland. Biol J Linnean Soc 112:442–449
Dufresnes C, Perrin N (2015) Effect of biogeographic history on population vulnerability in European amphibians. Conserv Biol 29:1235–1240
Dufresnes C, Brelsford A, Crnobrnja-Isailović J et al (2015a) Timeframe of speciation inferred from secondary contact zones in the European tree frog radiation (Hyla arborea group). BMC Evol Biol 15:155
Dufresnes C, Dubey S, Ghali K, et al (2015b) Introgressive hybridization of threatened European tree frogs (Hyla arborea) by introduced H. intermedia in Western Switzerland. Conserv Genet. In press
Earl DA, VonHoldt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361
Edgar P, Bird DR (2006) Action plan for the conservation of the crested newt Triturus cristatus species complex in Europe. The Herpetological Conservation Trust, Strasbourg
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol Bioinform 1:47–50
Ferrantin T (2007) Facteurs influençant l’occupation des étangs par des tritons crêtés: l’espèce autochtone Triturus cristatus, l’espèce invasive Triturus carnifex et l’hybride cristatus-carnifex et leurs distributions dans les cantons de Genève et Vaud (Suisse). Master Thesis, University of Neuchâtel, Neuchâtel, Switzerland
Fisher MC, Garner TWJ (2007) The relationship between the emergence of Batrachochytrium dendrobatidis, the international trade in amphibians and introduced amphibian species. Fungal Biol Rev 21:2–9
Fitzpatrick BM, Johnson JR, Kump DK et al (2010) Rapid spread of invasive genes into a threatened native species. Proc Natl Acad Sci 107:3606–3610
Franzen M, Gruber HJ, Heckes U (2002) An allochtonous Triturus carnifex population in southern Bavaria (Germany). Salamandra 38:149–154
Godinho R, Llaneza L, Blanco JC et al (2011) Genetic evidence for multiple events of hybridization between wolves and domestic dogs in the Iberian Peninsula. Mol Ecol 20:5154–5166
Grossenbacher K (1988) Atlas des amphibiens de Suisse. LSPN, Bâle
Grossenbacher K, Miaud C, Thiébaud J (2012) Triturus carnifex. In: Lescure J, de Massary JC (eds) Atlas des amphibiens et reptiles de France. Muséum national d’Histoire naturelle, Paris, p 272
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Jakobsson M, Rosenberg NA (2007) CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 23:1801–1806
Johnson JR, Fitzpatrick BM, Shaffer HB (2010) Retention of low-fitness genotypes over six decades of admixture between native and introduced tiger salamanders. BMC Evol Biol 10:147
Jombart T (2008) Adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24:1403–1405
Klopfstein S, Currat M, Excoffier L (2006) The fate of mutations surfing on the wave of a range expansion. Mol Biol Evol 23:482–490
Krupa AP, Jehle R, Dawson DA et al (2002) Microsatellite loci in the crested newt (Triturus cristatus) and their utility in other newt taxa. Conserv Genet 3:87–89
Kyle CJ, Davison A, Strobeck C (2003) Genetic structure of European pine martens (Martes martes), and evidence for introgression with M. americana in England. Conserv Genet 4:179–188
Laikre L, Schwartz MK, Waples RS, Ryman N (2010) Compromising genetic diversity in the wild: unmonitored large-scale release of plants and animals. Trends Ecol Evol 25:520–529
Maletzky A, Mikulíček P, Franzen M et al (2008) Hybridization and introgression between two species of crested newts (Triturus cristatus and T. carnifex) along contact zones in Germany and Austria: morphological and molecular data. Herpetol J 18:1–15
Maletzky A, Kaiser R, Mikulíček P (2010) Conservation genetics of crested newt species Triturus cristatus and T. carnifex within a contact zone in Central Europe: impact of interspecific introgression and gene flow. Diversity 2:28–46
Meilink WRM, Arntzen JW, van Delft JJCW, Wielstra B (2015) Genetic pollution of a threatened native crested newt species through hybridization with an invasive congener in the Netherlands. Biol Conserv 184:145–153
Meyer A, Zumbach S, Schmidt B, Monney J (2009) Les amphibiens et les reptiles de Suisse. Haupt Verlag Bern
Nylander JAA (2004) MrAIC.pl. Program distributed by the author. Evolutionary Biology Centre, Uppsala University, Uppsala, Sweden
Oliveira R, Randi E, Mattucci F et al (2015) Toward a genome-wide approach for detecting hybrids: informative SNPs to detect introgression between domestic cats and European wildcats (Felis silvestris). Heredity 115:195–205
Petit RJ, Excoffier L (2009) Gene flow and species delimitation. Trends Ecol Evol 24:386–393
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Randi E, Lucchini V (2002) Detecting rare introgression of domestic dog genes into wild wolf (Canis lupus) populations by Bayesian admixture analyses of microsatellite variation. Conserv Genet 3:31–45
Randi E, Hulva P, Fabbri E et al (2014) Multilocus detection of wolf x dog hybridization in italy, and guidelines for marker selection. PLoS ONE 9:e86409
Rosenberg NA (2003) DISTRUCT: a program for the graphical display of population structure. Mol Ecol Notes 4:137–138
Schmidt B, Zumbach S (2005) Liste rouge des espèces menacées en Suisse: Amphibiens. Haupt Verlag Bern, Bern
Senn HV, Barton NH, Goodman SJ et al (2010) Investigating temporal changes in hybridization and introgression in a predominantly bimodal hybridizing population of invasive sika (Cervus nippon) and native red deer (C. elaphus) on the Kintyre Peninsula, Scotland. Mol Ecol 19:910–924
Smith RK, Sutherland WJ (2014) Amphibian conservation: global evidence for the effects of interventions. Pelagic Publishing Ltd, Exeter
van Oosterhout C, Hutchinson WF, Wills DP et al (2004) Micro-Checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Wielstra B, Arntzen JW (2011) Unraveling the rapid radiation of crested newts (Triturus cristatus superspecies) using complete mitogenomic sequences. BMC Evol Biol 11:162
Wielstra B, Crnobrnja-Isailović J, Litvinchuk SN et al (2013) Tracing glacial refugia of Triturus newts based on mitochondrial DNA phylogeography and species distribution modeling. Front Zool 10:13
Wittenberg R (2005) An inventory of alien species and their threat to biodiversity and economy in Switzerland. CABI Bioscience Switzerland Centre report to the Swiss Agency for Environment, Forests and Landscape. The environment in practice n°0629. Federal Office for the Environment, Bern
Wyssmüller S (2007) Analyses expérimentales comparant la croissance et la survie des larves de triton crêté (Triturus cristatus) et des larves de l’hybride genevois (Triturus cristatus x Triturus carnifex). Master Thesis, University of Neuchâtel, Switzerland
Acknowledgments
We are grateful to P. Marchesi, S. Dubey, J.W. Arntzen, D. Canestrelli and P. Mikulicek for providing samples, to P. Buri and C. Metzger for helping in the field, M. Stöck for discussion, as well as to R. Sermier, N. Duvoisin and C. Stoffel for laboratory support. We also thank the Federal Office for the Environment (S. Pearson), the Conservation de la Faune du canton de Vaud (S. Sachot) and the Direction générale de la nature et du paysage du canton de Genève (G. Dändliker) for delivering authorizations and for funding. This study was also partly funded by the University of Lausanne.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dufresnes, C., Pellet, J., Bettinelli-Riccardi, S. et al. Massive genetic introgression in threatened northern crested newts (Triturus cristatus) by an invasive congener (T. carnifex) in Western Switzerland. Conserv Genet 17, 839–846 (2016). https://doi.org/10.1007/s10592-016-0825-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-016-0825-6