Abstract
The molecular mechanisms underlying sensitivity and resistance to radiotherapy is an area of active investigation and discovery as its clinical applications have the potential to improve cancer patients’ outcomes. In addition to the traditional pathways of radiation biology, our knowledge now includes molecular pathways of radiation sensitivity and resistance which have provided insights into potential targets for enhancing radiotherapy efficacy. Sensitivity to radiotherapy is influenced by the intricate interplay of various molecular mechanisms involved in DNA damage repair, apoptosis, cellular senescence, and epigenetics. Translationally, there have been several successful applications of this new knowledge into the clinic, such as biomarkers for improved response to chemo-radiation. New therapies to modify radiation response, such as the poly (ADP-ribose) polymerase (PARP) inhibitors, derived from research on DNA repair pathways leading to radiotherapy resistance, are being used clinically. In addition, p53-mediated pathways are critical for DNA damage related apoptosis, cellular senescence, and cell cycle arrest. As the understanding of genetic markers, molecular profiling, molecular imaging, and functional assays improve, these advances once translated clinically, will help propel modern radiation therapy towards more precise and individualized practices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiotherapy is the medical use of ionizing radiation to treat cancer, and in some instances benign diseases, that cannot be eradicated with surgery. Approximately 50% of all cancer patients require radiotherapy during treatment, and 40% of patients cured of cancer will have received radiotherapy [1]. The biological impact of high-energy radiation is mostly cytotoxic to cancer cells, and it is achieved through damage to the DNA double helix which impairs their proliferative ability by inducing double strand breaks and chromosomal aberrations. This oncological treatment is often used in conjunction with surgery and/or systemic therapies. An in-depth understanding of the radiobiological principles governing the response to radiotherapy forms the foundation for its clinical use, and it is fundamental to achieve maximum therapeutic effectiveness while limiting normal tissue toxicity. Modern radiotherapy involves the precise targeting of a localized target or tumor while minimizing collateral damage to surrounding nearby tissues. The radiotherapy dosage, fractionation schema and delivery techniques are customized depending on the type of cancer, its stage, anatomical location, size of the target and intent of treatment (palliative versus definitive/curative). Radiotherapy delivery methods include external beam radiation therapy, brachytherapy, and therapeutic radiopharmaceuticals.
Advances in the understanding of molecular mechanisms and genomic pathways of the sensitivity and resistance of radiotherapy are paving the way for the application of precision oncology principles in the radiation oncology field. Our understanding of radiobiology and repair processes following radiation has made enormous progress since the inception of the use of therapeutic radiation; in addition, the technology by which radiotherapy is delivered and the guidance systems have also been revolutionized over the past two decades using progressively more sophisticated imaging technology to precisely aim the radiation beam to a target. Treatment management paradigms have been traditionally based on clinical presentation and histopathology. Here, we highlight recent advances in our knowledge of the molecular mechanisms of sensitivity and resistance to radiotherapy that have paved the way for new biomarkers and the development of targeted therapies which help in selecting the most effective radiation treatment for cancer patients.
The traditional “5Rs” of radiobiology stand for Repair, Reoxygenation, Redistribution, Repopulation, and Radiosensitivity and are the basic principles that form the foundation of modern radiation therapy and contribute to our understanding of the molecular mechanisms of sensitivity and resistance to radiotherapy. Firstly, the principle of Repair refers to the cell’s ability to repair DNA damage caused by radiation primarily in the form of either single-strand or double-strand breaks. Precise DNA repair mechanisms have evolved over millions of years and are essential for cell survival; however, when they are defective, the ensuing dysregulation of these molecular mechanisms can contribute to exquisite radiosensitivity [2]. Secondly, Reoxygenation refers to the ability of oxygen to radiosensitize cancer cells by ‘fixing’ the DNA damage inflicted by radiation whereby molecular O2 donates an electron to a DNA base that has an unpaired electron as a result of radiation damage. In doing so, the oxygen atom destabilizes the DNA double helix even more, precipitating a break in the double helix backbone. Furthermore, an increase in oxygen levels in hypoxic regions of the tumor allows for oxygen-dependent DNA damage by reactive oxygen species production; in the absence of O2, cells are 3 times more resistant to the effect of radiation [3]. Thirdly, Redistribution, also known as reassortment, refers to changes in the cell cycle distribution after radiation exposure. Cell cycle distribution determines radiosensitivity and radioresistance as there are specific phases where the DNA is more vulnerable to damage or protected from radiation cytotoxic effects. Experiments in synchronously dividing cells have shown that mammalian cells are most sensitive to radiotherapy in the G2/M phases of the cell cycle and most resistant in the late S phase [4]. Repopulation refers to the proliferation of surviving cancer cells after each dose (fraction) of irradiation; in some cancers, repopulation accelerates after the 4th week of radiation and can contribute to regrowth of the tumor and eventual treatment failure. Finally, intrinsic properties of cancer cells, including dysregulation of repair pathways, give rise to their Radiosensitivity or Radioresistance compared to normal tissues [3]. Some cancers such as melanoma and renal cell cancer are deemed radiation resistant based on their response to standard doses of radiation, whereas other cancers such as lymphomas, germinomas and small cell lung cancer are considered radiosensitive. The reason why different cancer histologies respond differently to the same dose of radiotherapy is attributed to their intrinsic radiosensitivity, and only recently we have started to uncover the molecular determinants responsible for their ability to repair radiation damage which in turn determines their relative radiosensitivity or resistance.
The therapeutic window in clinical radiotherapy represents the range of radiation doses needed to achieve optimal Tumor Control Probability (TCP) while minimizing Normal Tissue Complication Probability (NTCP). TCP and NTCP are calculated using radiobiological models that consider various factors related to tumor response and normal tissue toxicity. The window between achieving a high TCP while maintaining the NTCP low at about 5% is usually narrow, thus achieving the right balance within this window is critical for designing effective and safe radiation treatment plans. The linear quadratic model in radiobiology is used to describe radiation response in tumoral and normal tissues [3]. This model, in general, holds for traditional, curative radiotherapy regimens where daily doses of 2 Gy are used for a course of radiation lasting 5–8 weeks, whereas it tends to break down when more modern forms of radiation are used such as Stereotactic Radiosurgery (SRS).
Identification of pathways or interventions that can sensitize tumor cells to smaller total dose of radiation is the focus of translational research with the goal of widening the “window” of the optimal therapeutic effects. The same can be done with the knowledge of resistance to radiotherapy, which could be applied clinically to protect normal tissues; this strategy would also increase the therapeutic window.
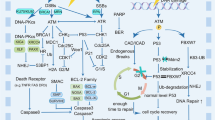
Below, we review key molecular pathways and processes influencing the response of mammalian cells to radiotherapy and are summarized in Fig. 1.
This is a graphical summarization of the known molecular mechanisms that contribute to the sensitivity and resistance of radiotherapy that ultimately determine treatment response. The complex interaction of the various molecular mechanisms involved in the processes described above coupled with the presence of hypoxia and the ability to repopulate have major implications for the radiosensitivity of tumors and the clinical practice of radiation therapy. This schematic was generated using BioRender.com.
Radiation sensitivity
Sensitivity to radiotherapy is primarily influenced by the intricate interplay of various molecular mechanisms involved in DNA damage repair. One critical DNA repair pathway primarily active during S and G2 phases of the cell cycle is the homologous recombination (HR) repair pathway. HR plays a pivotal role in repairing DNA double-strand breaks (DSBs), the most lethal form and a common type of damage induced by radiotherapy. Proteins such as RAD51 and BRCA1 are key players in HR and are crucial for the efficient and accurate repair of DSBs. Dysregulation or deficiencies in HR can result in compromised DNA repair capacity, leading to increased sensitivity to radiotherapy [5]. Conversely, increased expression of DNA repair genes (Rad51) has been associated with increased treatment resistance [6].
Another crucial molecular mechanism involved in sensitivity to radiotherapy is the activation of apoptotic pathways. Following exposure to ionizing radiation, cells with extensive DNA damage can undergo programmed cell death, known as apoptosis. The tumor suppressor protein p53 plays a central role in this process by inducing the expression of pro-apoptotic factors such as BAX and PUMA. Activation of the intrinsic apoptotic pathway results in the release of cytochrome c from mitochondria and subsequent activation of caspase cascades, ultimately leading to cell death. Alterations in the p53 pathway can impair apoptosis and therefore confer radioresistance (Fig. 2) [7].
Cellular senescence, a state of irreversible growth arrest, is also implicated in sensitivity to radiotherapy. Radiation-induced DNA damage can trigger a senescence response mediated by the p16INK4a-pRB and p19ARF-p53 pathways. Senescent cells exhibit altered gene expression patterns and secrete a range of factors collectively known as the senescence-associated secretory phenotype (SASP). The SASP components can influence the microenvironment, affecting neighboring cells and modulating the response to radiation. The presence of senescent cells can sensitize tumors to radiotherapy, as senescence-associated inflammation and immune responses can contribute to tumor cell elimination (Fig. 2) [8].
In addition to apoptosis and cellular senescence, p53 protein activation via phosphorylation in response to DNA damage also causes cell cycle arrest, an important step regulated by cell cycle checkpoints which allows repair of DNA damage before the cell enters mitosis [9]. The p53 protein is involved in cell cycle arrest after irradiation at the G2-M transitions thus allowing for repair of DNA damage, which in turn promotes resistance to radiotherapy [10]. Lastly, the p53 protein also has a role in opposing epithelial-to-mesenchymal transition (EMT), a process that has been correlated with resistance to radiotherapy and with a more aggressive phenotype; when p53 is mutated, its loss-of-function (or mutation) promotes cancer cell EMT by de-repressing Snail1 protein expression and activity, a process that eventually leads to radiation resistance (Fig. 2) [11]. Unfortunately, p53 mutations occur in more than 50% of cancers and thus it represents a common pathway of resistance [12].
Pathways of radiosensitivity via p53 activation, adapted from the KEGG (Kyoto Encyclopedia of Genes and Genomes). The p53 gene is a tumor suppressor gene, and the p53 protein is important for DNA repair, apoptosis, cellular senescence, and suppressing EMT. When EMT is dysregulated, or mutated, it leads to radiation resistance. This schematic was generated using BioRender.com
Additionally, gene expression of cancer cells plays a crucial role in determining sensitivity to radiotherapy. Epigenetic modifications such as DNA methylation, histone modifications, and the presence of non-coding RNAs are all regulators that influence gene expression patterns. Alterations in epigenetic markers can impact the expression of genes involved in DNA repair, cell cycle control, and apoptotic pathways, thereby influencing radiosensitivity. Targeting these epigenetic alterations holds potential for enhancing sensitivity to radiotherapy by modulating the expression of key genes involved in radiation response [13]. The methylation of the DNA repair enzyme O6-methylguanine-DNA methyltransferase (MGMT) is a prognostic and a predictive epigenetic biomarker in glioblastoma [14]. When the MGMT gene is silenced by promoter methylation, improved clinical outcomes are observed in patients with glioblastoma treated with radiation therapy and alkylating agents; whereas when the MGMT promoter is unmethylated, there is resistance due to increased DNA repair by MGMT [15].
Radiation resistance
Radiotherapy resistance is an intricate process that is caused by multiple mechanisms such as the presence of hypoxic cells within a tumor and increased capacity of DNA double-strand break repair. Identifying underlying mechanisms of radiotherapy resistance is an important area of research as better knowledge of the molecular pathways can lead to an improvement of treatments. One major driver of radiation resistance of cancer cells is enhanced DNA damage repair mechanisms. Radiation-induced DNA damage, such as double strand breaks, starts a cascade of signals in tumor cells that stimulate the expression levels of DNA repair enzymes and various survival promoting processes. An example of such upregulation of DNA repair enzymes is provided by a recent publication reporting an inverse correlation between Rad51 expression levels and survival of patients with glioblastoma; the higher the level of Rad51, the shorter the survival in this patient population [16]. Thus, increased repair capacity after radiation could explain the high local recurrence rate in patients with glioblastoma. Therefore, RAD51 is a potential therapeutic target that could be used to enhance the cytotoxic effect of radiation if selectively inhibited in tumor cells. Tumor hypoxia has long been recognized as a marker of radiation resistance and numerous attempts have been made to enrich tumors of O2 over the past 3 decades; however, only recently a practical approach to overcome hypoxia has been published which appears to be promising and non-toxic in glioblastoma, a tumor which commonly has a large hypoxic component [17].
In addition to double-strand break (DSB) repair, tumor cells can employ other DNA repair pathways, such as base excision repair (BER), nucleotide excision repair (NER), and mismatch repair (MMR). Since DSBs are the most lethal lesions induced by ionizing radiation, targeting these pathways is the most promising approach to overcome tumor resistance to radiotherapy [18, 19]. To bypass DNA damage induced by radiotherapy, tumor cells not only use enhanced DNA repair capacity but also increase DNA damage tolerance allowing them to continue proliferating. The X-ray repair cross-complementing 1 (XRCC1) is an example of such mitigation strategy used by cancer cells; XRCC1 plays an important role in BER and is involved with single-strand breast (SSB) repair. High expression of XRCC1 has been associated with poorer survival in head and neck cancer and was seen primarily in the subset of patients who received chemoradiation [20]. Furthermore, XRCC1 gene polymorphism has been associated with increased susceptibility to radiation-induced toxicities, such as radiation dermatitis and oral mucositis [21, 22]. However, expression of XRCC1 in tumor cells is not always a predictor of pro-survival ability (radioresistance) in all tumor cells; for example, in bladder cancer tumor tissue samples, high levels of XRCC1 expression were associated with improved cancer-specific survival [23].
Another protein that supports the survival of tumor cells after radiothearpy is replication protein A (RPA), which is crucial for genomic stability by participating in DBS repair. RPA has 3 subunits, and all of them are expressed at high levels in glioblastoma specimens, a tumor notorious for its poor response to radiotherapy. In vitro studies have shown that targeting one of the RPA subunits, RPA70, significantly impairs glioblastoma cancer stem-like cells. A small molecular inhibitor, called HAMNO, targets the interaction of RPA70 and ataxia telangiectasia and Rad3-related protein (ATR) [24]. Pre-clinical studies are ongoing with RPA inhibitors and their potential as a DNA damage response (DDR) inhibitor for clinical use [25].
The poly (ADP-ribose) polymerase (PARP) inhibitors are a class of DNA damage response (DDR) inhibitors that has already been translated in clinical use over the past few years. PARPs are involved in numerous cellular processes, including DNA repair, DNA replication, transcription, and post-translational modification of histones and other nuclear proteins. PARP-1 is the primary member of the PARP family and facilitates DNA damage response by sensing and modifying DNA free ends so that repair can commence. Olaparib was the first inhibitor approved in 2014; since then, multiple other PARP inhibitors are now in clinical use for patients with ovarian, breast and prostate cancers who have defects in the homologous recombination (HR) DNA repair pathways and BRCA mutations [26]. PARP inhibitors have been shown to enhance response to ionizing radiation as well as chemotherapies in BRCA-proficient NSCLC cells [27]. Its ability to sensitize radiation resistant tumors is already being tested in clinical trails.
Finally, the tumor microenvironment, commonly hypoxic in multiple cancers, reduces oxygen-dependent DNA damage typically induced by photon radiotherapy. Hypoxia is caused by aberrant cell proliferation and poor tumor vascularization. Heterogenous oxygen microenvironments and cancer cell-mediated inflammatory and signaling also impart cellular changes in the microenvironment, specifically tumor-associated macrophages (TAMs) and cancer-associated fibroblasts (CAFs). In certain tumor types, TAMs enhance radioresistance by inducing tumor growth (increased re-population). In addition, the development of CAFs is stimulated by cancer cell signaling and hypoxic tumor microenvironment [28]. These CAFs also increase radioresistance by secreting signaling molecules such as epidermal growth factor (EGF) that lead to proliferation and survival of cancer cells after irradiation (Fig. 3) [29].
Technical advances leading to an increase in the tumoricidal effects of radiation
Advances in radiotherapy modalities and techniques that have occurred over the past few decades include particle beam radiotherapy, therapeutic radionuclides, and changes in fractionation regimens. Particle beam radiotherapy refers to the use of charged particles, such as protons or carbon ions. Heavier ionizing particles than photons have the advantage of reduced damage to surrounding healthy tissues due to increased sparing of healthy tissue from the Bragg peak phenomenon while also increasing the ablative nature of the radiation-induced damage. Although there are few clinical trials showing a difference in effectiveness between particle and photon therapies, several randomized controlled comparative clinical trials are ongoing [30].
Radiopharmaceuticals can also be used to overcome radiation resistance by virtue of their targeted delivery. Radioligand therapies allow for the delivery of targeted treatment to cells harboring tumor specific molecular targets. With this form of therapy, radionuclides are attached to a monoclonal antibody targeting a specific molecular target on the cell surface, allowing the radiopharmaceutical molecule to concentrate in the tumor cells. For example, a recent breakthrough in the treatment of castration-resistant prostate cancer has emerged with 177Lu-PSMA-617, which has been shown to improve overall survival with delivery of a dose of 7.4 GBq (200 mCi) once every 6 weeks for 4–6 cycles [31]. In contrast to external beam radiotherapy, radiopharmaceuticals have longer exposure times and lower absorbed dose rate [32]. Among many other investigational radiopharmaceutical agents, the fibroblast activation protein (FAP) is a promising target for radiopharmaceutical therapy. FAP is specifically expressed in many cancer-associated fibroblasts, and this protein differentiates the CAFs from the surrounding normal fibroblasts. FAP has been tagged with 177Lu and is in the early clinical trial phase for metastatic radioactive iodine refractory thyroid cancer patients with progressive disease after treatment with tyrosine kinase inhibitors [33].
Finally, with the advent of stereotactic technology and CT guidance, various hypo-fractionated radiation schemas have been adopted over the past decade; these abbreviated courses of radiation deliver much higher biological doses of radiation than conventionally fractionated schemas thus achieving cumulative biological effective doses (BED) higher than 100 Gy. This higher biological dose increases the ability to overcome radiation resistance thus increasing the chance of ablating the target and improving the efficacy of the radiotherapy.
Spatially fractionated radiotherapy (SFRT) is another technical advance in external beam radiotherapy delivery that allows for strategic placement of heterogenous high dose of radiation within tumor volumes increasing tumoricidal effects with minimal increase in normal tissue toxicity. In contrast to spatial heterogeneity, flash radiotherapy (FLASH-RT) alters the dose rate to ultrahigh levels which appear to spare normal tissues. FLASH-RT delivers radiation at a rate of 40 Gy/second while current treatments are delivered at a rate of ~ 0.01 Gy/second [34]. FLASH-RT has the potential to increase tumor responses while significantly sparing normal tissue [35].
Conclusions
Radiotherapy continues to have a major role in the treatment of cancer. However, there are cancers where a higher local control is desirable, such glioblastoma and pancreatic cancer. Understanding the molecular mechanisms leading to radiation sensitivity or resistance is important for improving treatment efficacy and patient outcomes in radiation resistant cancers. Advances in radiobiology and an increased understanding of molecular pathways have provided insights into potential targets for enhancing radiotherapy. In addition, greater understanding of genetic markers, increased use of molecular profiling, improved molecular imaging, and functional assays are providing insights on predicting treatment response. These advances are all taking us towards more precise and individualized clinical care. Ongoing basic research and clinical translational trials are vital for harnessing these discoveries and implementing precision oncology principles in radiation oncology, ultimately leading to better treatment strategies and improved patient survival rates.
References
Baskar R, Lee KA, Yeo R, Yeoh KW (2012) Cancer and radiation therapy: current advances and future directions. Int J Med Sci 9(3):193–199. https://doi.org/10.7150/ijms.3635Epub 2012 Feb 27. PMID: 22408567; PMCID: PMC3298009
Arenz A, Ziemann F, Mayer C et al (2014) Increased radiosensitivity of HPV-positive head and neck cancer cell lines due to cell cycle dysregulation and induction of apoptosis. Strahlenther Onkol 190:839–846. https://doi.org/10.1007/s00066-014-0605-5
Hall E, Giaccia A (2018) Radiobiology for Radiologist. Lippincott Williams & Wilkins, Philadelphia
Sinclair WK (1968) Cyclic X-ray responses in mammalian cells in vitro. Radiat Res. 2012;178(2):AV112-AV124. https://doi.org/10.1667/rrav09.1
Bouwman P, Jonkers J (2012) The effects of deregulated DNA damage signaling on cancer chemotherapy response and resistance. Nat Rev Cancer 12(9):587–598. https://doi.org/10.1038/nrc3342
Vispé S, Cazaux C, Lesca C, Defais M Overexpression of Rad51 protein stimulates homologous recombination and increases resistance of mammalian cells to ionizing radiation, Nucleic Acids Research, Volume 26, Issue 12, 1 June 1998, Pages 2859–2864, https://doi.org/10.1093/nar/26.12.2859
Vousden KH, Prives C (2009) Blinded by the light: the growing complexity of p53. Cell 137(3):413–431. https://doi.org/10.1016/j.cell.2009.04.037
Rodier F, Campisi J (2011) Four faces of cellular senescence. J Cell Biol 192(4):547–556. https://doi.org/10.1083/jcb.201009094
Bieging KT, Mello SS, Attardi LD (2014) Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer 14(5):359–370. https://doi.org/10.1038/nrc3711
Pawlik TM, Keyomarsi K (2004) Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys 59(4):928–942. https://doi.org/10.1016/j.ijrobp.2004.03.005
Kim NH, Kim HS, Li XY et al (2011) A p53/miRNA-34 axis regulates Snail1-dependent cancer cell epithelial-mesenchymal transition. J Cell Biol 195(3):417–433. https://doi.org/10.1083/jcb.201103097
Rivlin N, Brosh R, Oren M, Rotter V (2011) Mutations in the p53 tumor suppressor gene: important milestones at the various steps of Tumorigenesis. Genes Cancer 2(4):466–474. https://doi.org/10.1177/1947601911408889PMID: 21779514; PMCID: PMC3135636
Zhang L, Lu Q, Chang C (2020) Epigenetics in Health and Disease. Adv Exp Med Biol 1253:3–55. https://doi.org/10.1007/978-981-15-3449-2_1
Hegi ME, Liu L, Herman JG et al (2008) Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol 26(25):4189–4199. https://doi.org/10.1200/JCO.2007.11.5964
Hegi ME, Diserens AC, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Morrison C, Weterings E, Mahadevan D, Sanan A, Weinand M, Stea B (2021) Expression levels of RAD51 inversely correlate with survival of Glioblastoma patients. Cancers 13(21):5358. https://doi.org/10.3390/cancers13215358
Lickliter JD, Ruben J, Kichenadasse G, Jennens R, Gzell C, Mason RP, Zhou H, Becker J, Unger E, Stea B (2023) Dodecafluoropentane Emulsion as a Radiosensitizer in Glioblastoma Multiforme. Cancer Res Commun 3(8):1607–1614. https://doi.org/10.1158/2767-9764.CRC-22-0433PMID: 37609003; PMCID: PMC10441549
Liu YP, Zheng CC, Huang YN, He ML, Xu WW, Li B (2020) Molecular mechanisms of chemo- and radiotherapy resistance and the potential implications for cancer treatment. Med Comm 2021;2(3):315–340. https://doi.org/10.1002/mco2.55
Huang RX, Zhou PK (2020) DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct Target Ther 5(1):60. https://doi.org/10.1038/s41392-020-0150-x. Published 2020 May 1
Ang MK, Patel MR, Yin XY et al (2011) High XRCC1 protein expression is associated with poorer survival in patients with head and neck squamous cell carcinoma. Clin Cancer Res 17(20):6542–6552. https://doi.org/10.1158/1078-0432.CCR-10-1604
Terrazzino S, La Mattina P, Masini L et al (2012) Common variants of eNOS and XRCC1 genes may predict acute skin toxicity in breast cancer patients receiving radiotherapy after breast conserving surgery. Radiother Oncol 103(2):199–205. https://doi.org/10.1016/j.radonc.2011.12.002
Osti MF, Nicosia L, Agolli L et al (2017) Potential role of single nucleotide polymorphisms of XRCC1, XRCC3, and RAD51 in Predicting Acute toxicity in rectal Cancer patients treated with preoperative Radiochemotherapy. Am J Clin Oncol 40(6):535–542. https://doi.org/10.1097/COC.0000000000000182
Sak SC, Harnden P, Johnston CF, Paul AB, Kiltie AE (2005) APE1 and XRCC1 protein expression levels predict cancer-specific survival following radical radiotherapy in bladder cancer. Clin Cancer Res 11(17):6205–6211. https://doi.org/10.1158/1078-0432.CCR-05-0045
Pedersen H, Adanma Obara E, Elbæk KJ, Vitting-Serup K, Hamerlik P, Replication Protein A (RPA) Mediates Radio-Resistance of Glioblastoma Cancer Stem-Like Cells (eds) (2020) Int J Mol Sci. 21(5):1588. Published 2020 Feb 26. https://doi.org/10.3390/ijms21051588
VanderVere-Carozza PS, Gavande NS, Jalal SI et al (2022) Vivo targeting replication protein A for Cancer Therapy. Front Oncol 12:826655 Published 2022 Feb 18. https://doi.org/10.3389/fonc.2022.826655
Hunia J, Gawalski K, Szredzka A, Suskiewicz MJ, Nowis D (2022) The potential of PARP inhibitors in targeted cancer therapy and immunotherapy. Front Mol Biosci 9:1073797 Published 2022 Dec 1. https://doi.org/10.3389/fmolb.2022.1073797
Ryu H, Kim HJ, Song JY et al (2019) A small compound KJ-28d enhances the sensitivity of Non-small Cell Lung Cancer to Radio- and chemotherapy. Int J Mol Sci 20(23):6026 Published 2019 Nov 29. https://doi.org/10.3390/ijms20236026
Suwa T, Kobayashi M, Nam JM, Harada H (2021) Tumor microenvironment and radioresistance. Exp Mol Med 53(6):1029–1035. https://doi.org/10.1038/s12276-021-00640-9
Chu TY, Yang JT, Huang TH, Liu HW (2014) Crosstalk with cancer-associated fibroblasts increases the growth and radiation survival of cervical cancer cells. Radiat Res. 181(5):540–7. https://doi.org/10.1667/RR13583.1. Epub 2014 May 1. PMID: 24785588
Chen Z, Dominello MM, Joiner MC, Burmeister JW (2023) Proton versus photon radiation therapy: A clinical review. Front Oncol. 13:1133909. Published 2023 Mar 29. https://doi.org/10.3389/fonc.2023.1133909
Sartor O, de Bono J, Chi KN et al (2021) Lutetium-177-PSMA-617 for metastatic castration-resistant prostate Cancer. N Engl J Med 385(12):1091–1103. https://doi.org/10.1056/NEJMoa2107322
English KK, Knox S, Graves SA, Kiess AP (2022) Basics of physics and Radiobiology for Radiopharmaceutical therapies. Pract Radiat Oncol 12(4):289–293. https://doi.org/10.1016/j.prro.2022.04.004
Evaluation of 177 Lu-DOTA-EB-FAPI in patients with metastatic Radioactive Iodine refractory thyroid Cancer. ClinicalTrials.gov Identifier: NCT05410821
Borghini A, Vecoli C, Labate L, Panetta D, Andreassi MG, Gizzi LA (2022) FLASH ultra-high dose rates in radiotherapy: preclinical and radiobiological evidence. Int J Radiat Biol 98(2):127–135. https://doi.org/10.1080/09553002.2022.2009143
Favaudon V, Caplier L, Monceau V et al (2014) Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice [published correction appears in Sci Transl Med. 2019;11(523)]. Sci Transl Med 6(245):245ra93. https://doi.org/10.1126/scitranslmed.3008973
Funding
None.
Author information
Authors and Affiliations
Contributions
BS conceived manuscript. JX and BS wrote the manuscript. All authors reviewed final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xing, J.L., Stea, B. Molecular mechanisms of sensitivity and resistance to radiotherapy. Clin Exp Metastasis 41, 517–524 (2024). https://doi.org/10.1007/s10585-023-10260-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-023-10260-4