Abstract
Diagnosing malignant pleural effusions (MPE) is challenging when patients lack a history of cancer and cytopathology does not detect malignant cells in pleural effusions (PE). We investigated whether a systematic analysis of PE by flow cytometry immunophenotyping (FCI) had any impact on the diagnostic yield of MPE. Over 7 years, 570 samples from patients with clinical suspicion of MPE were submitted for the FCI study. To screen for epithelial malignancies, a 3-color FCI high sensitivity assay was used. The FCI results, qualified as “malignant” (FCI+) or “non-malignant” (FCI-), were compared to integrated definitive diagnosis established by clinicians based on all available information. MPE was finally diagnosed in 182 samples and FCI detected 141/182 (77.5%). Morphology further confirmed FCI findings by cytopathology detection of malignant cells in PE (n = 91) or histopathology (n = 29). Imaging tests and clinical history supported the diagnosis in the remaining samples. The median percentage of malignant cells was 6.5% for lymphoma and 0.23% for MPE secondary to epithelial cell malignancies. FCI identified a significantly lower percentage of EpCAM+ cells in cytopathology-negative MPE than in cytopathology-positive cases (0.02% vs. 1%; p < 0.0001). Interestingly, 29/52 MPE (55.8%) where FCI alerted of the presence of malignant cells were new diagnosis of cancer. Overall, FCI correctly diagnosed 456/522 samples (87.4%) suitable for comparison with cytopathology. These findings show that high sensitivity FCI significantly increases the diagnostic yield of MPE. Early detection of FCI + cases accelerates the diagnostic pathway of unsuspected MPE, thus supporting its implementation in clinical diagnostic work-up as a diagnostic tool.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant pleural effusions (MPE) are usually a late manifestation of a disseminated disease in a patient with a known history of cancer. However, between 10 and 15% of MPE are the first sign of an underlying tumor, making this diagnosis particularly challenging. Reported sensitivity of pleural fluid cytopathology ranges from 45 to 60%, influenced by the experience of the cytopathologist, volume of samples received or type of tumor [1].To date, biomarkers tested for MPE have not reached enough evidence to support their routine measurement in clinical practice [2]. Clinical guidelines recommend the use of flow cytometry immunophenotyping (FCI) for very selected pleural effusions (PE) with high suspicion of involvement by lymphoma [3]. However, many authors have also used FCI to detect infiltration of non-hematological neoplasms in body fluids [4,5,6,7,8,9,10,11]. Considering that lymphoma accounts for less than 10% of MPE, FCI would offer an opportunity as part of the diagnostic algorithms for PE given its function to detect epithelial cell malignancies through the identification of the epithelial cell adhesion molecule (EpCAM) [12]. This prospective 7-year study is the result of investigating whether implementing a systematic FCI evaluation of PE samples with clinical suspicion of malignancy would have an impact on the diagnostic yield of MPE.
Materials and methods
Study design
Between 2015 and 2022, 1,750 PE samples were recorded at our hospital. Patients with clinical suspicion of malignancy were submitted to a pleural tap to study PE using biochemistry, cytopathology and cell culture. A PE sample was also systematically sent for FCI study.
Data from patients included: age at evaluation, gender, tumor history, imaging diagnostic tests (radiography, computerized tomography, PET), cytopathology and/or histopathology data. Cytopathological analysis was performed by conventional microscopy and findings were reported according to the international system for reporting serous fluid cytology [13]. All PE samples were examined using smears. Immunohistochemistry of cell blocks was only performed for samples with a high percentage of malignant cells.
The final diagnosis of PE was established by clinicians on the basis of all available information, and this integrated diagnosis was considered as the “gold standard”. Three clinical categories of PE were established: MPE, paramalignant effusions and PE negative for malignancy [14].
MPE was established when tumor cells were detected, either in PE by cytology or in a tissue biopsy sample. In the present study, samples reported by cytology as “malignant” or “suspicious for malignancy” were considered MPE. In turn, some cases with a negative cytopathology result were finally diagnosed as MPE on the basis of an integrated diagnosis, including histology, imaging tests and clinical impression of malignancy.
The term “paramalignant effusions” was reserved for those effusions thought to be related to an active primary neoplastic disease but without demonstration of malignant cells in PE or pleural tissue.
PE was considered “negative for malignancy” when morphology and the whole clinical information reasonably ruled out a neoplasm after a follow-up of at least 6 months. Particularly, in patients with a previous history of tumor, PE was classified as negative for malignancy when there was no data of tumor progression and the development of PE could be explained by a specific etiology such as: congestive heart failure, infection, surgery, renal or hepatic disease, and so on.
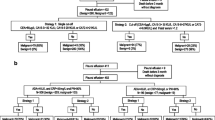
FCI results were either reported as “non-malignant”, “malignant”, or “suboptimal sample”. The results were then compared with the final diagnosis of PE established by clinicians. An overview of the design of the study is summarized in Fig. 1. All clinical procedures followed the standards set by guidelines of the latest Declaration of Helsinki.
Overview of the study design. PE: Pleural effusion; FCI: flow cytometry immunophenotyping; MPE: malignant pleural effusion; FCI+: PE classified as malignant by flow cytometry immunophenotyping; FCI-: PE classified as non-malignant by flow cytometry immunophenotyping. Numbers in parentheses refer to PE samples
FCI studies
Fresh PE samples were processed within 24 h from reception, and most within the first 3 h after collection. After cell count and centrifugation, fluid was removed to get a cell-rich sample. Whenever it was possible, the cell pellet was resuspended in phosphate buffered saline at a final concentration of 6–8 × 106 cells/200 microliters. For cases with low cellcount, all cells were stained to cover the acquisition of the largest possible number of events in the flow cytometer.
The most likely clinical diagnosis determined the panel of monoclonal antibodies (mAb) to be used. For most samples, the following 3-color “epithelial cell” tube was used: anti-EpCAM FITC (clone Ber-EP4, DAKO), anti-CD45 PerCP (clone 2D1, BDB), and anti-CD200 APC (clone OX104, e-Bioscience). For samples with suspicion of hematological malignancy, an 8-color combination of mAb was used [15]. In all cases, a direct immunofluorescence stain-and-then-lyse technique was applied [5].
Cells were acquired in a FACSCanto II flow cytometer (Becton Dickinson, San Jose, CA) using the FACSDiva software (Becton Dickinson). Two different types of calibration beads (BD FACSDiva™ CS&T IVD Beads, and 8-peak Rainbow beads, Spherotech, Lake Forest, IL), were daily used to set up the flow cytometer and to perform daily quality control. To minimize sample contamination, a careful cleaning of the fluidic system was performed prior to acquisition. The parameter “time” was used to discard problems during the acquisition due to clogs. To screen for epithelial malignancies, we acquired the whole sample volume or at least 5 × 106 events. For samples with suspicion of lymphoma, 1 × 106 events were acquired.
Data analysis was performed with the Infinicyt software program (Cytognos SL, Salamanca, Spain). Figure 2 summarizes the strategy used to identify epithelial cells. In brief, mesothelial cells were used as an internal negative control to set the cut-off for EpCAM and CD45 expression. Lymphocytes were used as an internal positive control of CD45. For detecting epithelial cells the gating strategy was focused on CD45neg events where epithelial cells, if present, would overlap the CD45neg expression of mesothelial cells. In hematological malignancies, FCI was set to search for abnormal patterns of antigen expression in leukocytes [16]. Neoplastic cells were referred to total cellularity (leukocytes and non- hematological cells) [5].
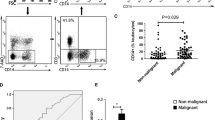
FCI strategy used to identify epithelial cells. The analysis was performed in 2 steps. Panel A shows the sequential steps for the identification of mesothelial cells (MC, depicted in pink) and lymphocytes (depicted in violet). Both populations were used as internal negative controls for EpCAM expression. Panel B describes the sequential steps for definition of EpCAM+ cells using the monoclonal antibody anti-EpCAM FITC (clone Ber-EP4): after a wide selection of Ber-EP4 positive events, 2 additional gates were performed to include only CD45 negative cells. MC established the cutoff for any CD45 negative population, and lymphocytes were markers of positive CD45 expression. Finally, only those Ber-EP4 positive events with the same or higher forward and side scatter pattern than lymphocytes were selected. Panel C depicts the result of the whole analysis after discarding all the events that did not fulfill the requirements of a Ber-EP4 positive population. In this specific case, the epithelial cell population (depicted in blue) was negative for CD200 expression
The limit of detection (LOD) and lower limit of quantification (LLOQ) defined the level of sensitivity of the FCI assay [17]. In brief, 30 clustered events were the cutoff to classify a rare population as detectable, and 50 events were the minimum value to define a population as quantifiable. Therefore, LOD and LLOQ were not fixed values and varied according to whole collected events. FCI qualified a sample as malignant when the percentage of EpCAM+ cells or hematological malignant cells was above the LLOQ. All FCI results were blinded to cytopathologist analyses.
Statistics
Sensitivity, specificity, positive and negative predictive values of FCI were calculated for PE samples that integrated diagnosis classified as MPE or negative for malignancy; paramalignant cases were excluded. Relationships between categorical variables were performed using χ2 test or Fisher’s exact test.Statistical analyses were carried out using GraphPad Prism Viewer 9.4.1 software. All p-values represented were two-sided and p-values < 0.05 were considered statistically significant.
Results
Study cohort and final diagnosis of PE
Based on clinical suspicion of malignancy, 570 out of the 1,750 PE samples (32.6%) collected in our hospital in this 7-year study were sent for FCI evaluation. Only samples with a proper FCI and cytopathology result were considered for analyses. Samples not suitable for comparison included those PE not sent for cytopathological analysis (n = 18), samples classified by cytopathology as “nondiagnostic” (n = 9) or “atypia of undetermined significance” [13] (n = 8), and suboptimal samples not adequate for FCI evaluation (n = 13) (Supplementary Information 1). At the end, the study included 522 out of the 570 samples (91.6%) and involved 467 patients. At the time of the study, no history of malignancy had been reported in most of them (n = 285/467; 61%) (Table 1).
Integrated diagnosis classified 318/522 samples (60.9%) as negative for malignancy, 22 as paramalignant (4.2%), and 182 as MPE (34.9%) (Fig. 1). Lymphoma was the only hematological malignancy causing MPE (24/182 cases; 13.2%), mesothelioma was involved in 12 MPE (6.6%), and epithelial cell neoplasms in 140 (76.9%) (Table 2). Lung and pleural tumors were responsible for most MPE (110 vs.48 from other localizations), and for most new diagnosis of cancer (n = 76). Gastrointestinal tract tumors (n = 14) and breast adenocarcinoma (n = 11) were the most frequent non-lung tumors developing MPE (Table 3).
Correlation of FCI findings and definitive diagnosis
Median sample volume received for FCI study was 15 ml (IQR1:10; IQR3: 20), and median cell count was 1 cell/1 × 10− 3 L (IQR1:0.4; IQR3: 2.7). FCI results were reported independently of cytopathology, and median time between the reception of the PE and FCI reporting was 12 h. Besides, whenever FCI detected malignant cells, the result was reported to clinicians within 3 h after sample reception. Overall, FCI correctly diagnosed 415/467 patients (88.9%) and 456/522 samples (87.4%). Interestingly, FCI also identified 95.7% of MPE without a simultaneous cytological study and those classified by cytology as “acellular” (Supplementary Information 1).
Malignant FCI cases
FCI identified 144 samples as malignant (FCI+) and 141/144 (97.9%) were diagnosed as MPE (77.5% of all MPE). In contrast, 3 patients where FCI detected low percentages of EpCAM+ cells were finally considered as PE negative for malignancy. One patient (0.04% EpCAM+ cells) was diagnosed with pulmonary tuberculosis. Another patient (0.014% EpCAM+ cells) presented an advanced alcoholic hepatitis with no data of malignancy in pleural biopsy. Interestingly, this patient was diagnosed of gastric adenocarcinoma without metastatic dissemination two months later. The third patient (0.011% EpCAM+ cells) was receiving therapy for a disseminated thyroid papillary cancer, and needed a pericardial drainage for a concomitant pericardial effusion. After a ten-month follow-up, neither PE nor progression of the disease was documented in this third patient.
For the remaining 141 FCI + cases, the diagnosis of MPE was corroborated by other techniques. In most cases (n = 89), cytopathology detected malignant cells in the same pleural tap, and a second pleural tap was necessary to detect malignancy in 2 patients. Histopathology (n = 29), imaging tests (n = 11) or consistent clinical history (n = 6) confirmed FCI + results. Finally, a low-grade lymphoma was detected in the remaining 4 samples from two patients.
Regarding diagnosis, FCI detected 20/24 lymphoma (83.3%), and 121/158 (76.6%) solid neoplasms, most of which (117/121; 96.7%) were epithelial cell neoplasms. There was no association between FCI + cases, previous history of cancer or primary tumor localization (Table 3). Of note, 29/52 MPE (55.8%) which were only detected by FCI were new diagnosis of cancer; 4 were NHL and 20/25 solid tumors were located in the lung (Table 3). FCI detected malignant cells in all histological lung subtypes, and statistically significant differences were observed among them (Fig. 3). As expected mesothelioma was the tumor with a fewer number of FCI + cases, followed by squamous cell carcinoma (SSC). Median percentage of EpCAM+ cells detected by FCI was 0.23% (IQR1: 0.031%- IQR3: 3.75%). In 21/117 samples (18%) the percentage of EpCAM+ cells was ≤ 0.01%. As shown in Fig. 4, the intensity of EpCAM expression was heterogeneous among histological subtypes and also within individual tumors.
Description of FCI results in MPE secondary to mesothelioma and different histological lung subtypes. Green boxes reflect MPE detected by FCI (FCI+). Negative data (depicted in red) represent the number of cases not detected by FCI (FCI-). Dashed bars include cases with a known previous history of cancer. NSCC-NOS: non-small cell cancer not otherwise specified. NET: neuroendocrine tumors included 13 small-cell carcinoma, 1 non-small cell NET and 1 atypical carcinoid tumor
Examples of EpCAM expression in different tumor subtypes plotted as median fluorescence intensity with boxplots. The line inside the box is the median value, the upper and lower box limits show the 75th and 25th percentiles, and whiskers indicate the minimum and maximal level of expression. Examples of 3 lung adenocarcinoma (boxplots 1–3 depicted in blue), 2 neuroendocrine tumors (boxplots 4–5, depicted in green), 1 squamous cell carcinoma (boxplot 6 depicted in brown), 1 gastric adenocarcinoma (boxplot 7 depicted in red), 1 breast adenocarcinoma (boxplot 8 depicted in pink), and 1 bowel carcinoma (boxplot 9 depicted in yellow)
Median percentage of lymphoma in MPE was 6.5% (IQR1: 0.2%- IQR3: 63.1%), and high-grade lymphoma presented the highest percentage of infiltration (median 38.5%; IQR1: 3.3%- IQR3: 78.3%). These patients started therapy after diagnosis compared to only 6/8 patients diagnosed with low-grade lymphoma.
Non-malignant FCI cases
FCI classified 378 samples as non-malignant (FCI-): 22 were finally classified as paramalignant, and 315 (83.3%) correlated with the clinical category of “PE negative for malignancy”. One hundred and five samples (33.3%) were from 88 patients with previous history of cancer. The remaining 41 FCI- cases, which belonged to 38 patients, were finally classified as MPE. An explanation for FCI- results could be obtained in 18/38 patients (47.4%): histopathology identified 1 synovial sarcoma and 10 mesothelioma, 3 patients had a percentage of EpCAM+ cells below the LOD, 1 clear renal carcinoma presented a negative expression of EpCAM according to histopathology, and 2 MPE secondary to diffuse large B-cell lymphoma lacked B-cells. These last two patients were finally diagnosed by pleural biopsy and imaging techniques. Another patient which was initially evaluated to discard epithelial cell neoplasms was finally diagnosed with primary effusion lymphoma (PEL). Seventeen days later a second FCI study focused on lymphocytes detected lymphoma. In the remaining 21 patients, the diagnosis of MPE was established on the basis of biopsy (n = 10) or PET/TC images (n = 7), but 4 cases presented a very bad performance and clinicians concluded progression of thymoma (n = 1) and high suspicion of malignancy (n = 3).
Correlation of FCI and cytology findings
Since FCI and cytopathology were the primary tools to evaluate PE, we aimed to compare both techniques. FCI results significantly correlated with morphological results diagnosis: 462/522 studies (88.5%) were concordant, while 60/522 (11.5%) were discordant (p < 0.0001). MPE was identified by both techniques in 89/182 (48.9%) cases, but neither cytology nor FCI detected 36/182 (19.8%) MPE (Table 2). As detailed in Table 4, the diagnostic yield of the FCI study on its own was high, but when cytology and FCI were considered together, the sensitivity and negative predictive value of the PE study increased to 80% and 90%, respectively.
In an attempt to explain the differences between the FCI and cytopathology findings, we focused on epithelial cell tumors, and compared MPE where both FCI and cytopathology were positive for malignancy (n = 80) and MPE only detected by FCI (n = 37) (Table 2; Fig. 5). There was no association with sample volume, cell count or median LOD and LLOQ, but statistically significant differences were observed in the median percentage of EpCAM+ cells detected by FCI in cytopathology-positive MPE (1%) and cytopathology-negative MPE (0.02%) p < 0.001. Interestingly, a significant higher number of MPE with ≤ 0.01% EpCAM+ cells was found in the group of cases not detected by cytopathology (17/37 (46%) compared to the cytopathology positive group (4/80; 5%) (p < 0.001).
Percentages of EpCAM+ cells detected by FCI in MPE samples classified as positive or negative for malignancy by cytopathology. The top and bottom lines represent the 75th and 25th percentiles, and the line in the middle represents the median value. FCI+: PE classified as “malignant” by flow cytometry immunophenotyping. Cy+: PE classified as “malignant” by cytology. Cy-: PE classified as “non-malignant” by cytology. LOD: limit of detection; LLOQ: lower limit of quantification. ns: statistically non-significant
Discussion
Cytopathology plays a central role in the diagnosis of MPE, but a diagnosis is delayed when malignant cells are not detected in PE [1]. In line with previous studies [8, 18, 5] our large series confirms that by using FCI, the chances of detecting MPE in a single tap increases by 28.6%. The targets of this FCI approach are lymphoma and epithelial cell malignancies, but as described in one patient in our series, unusual mesotheliomas may show weak EpCAM expression detected by histology [19, 20] or FCI [5, 6]. A high level of expression of CD200 and CD81, expression of CD73, and absence of CD45 and EpCAM are normal features of mesothelial cells. A number of additional molecules already used by immunohistochemistry [11, 19, 21, 22] particularly, CD146 [23, 24] and desmin [8], might help FCI to discriminate between reactive mesothelial cells and mesothelioma.
There are two main advantages of using FCI to evaluate PE samples suspicious of malignancy. According to our study, a median PE volume of 15 ml is enough to get reliable FCI results, which is lower than the recommended 50–75 ml for proper cytological evaluation [13]. The present finding would support considering FCI results in PE samples classified by cytopathologists as “non-diagnostic” [7, 8]. The second advantage is that the high sensitive FCI protocol reproduces the strategy to quantify measurable residual disease in hematological malignancies [25], and rescues MPE not detected by cytopathology. By staining and then analyzing a high number of cells, FCI may reach detection levels down to 1 malignant cell per 100,000 total cells. While other authors applied different cutoff levels of BerEP4 expression to separate benign from malignant effusions [7, 9], this high sensitive protocol would solve the limitation of cytology when malignant cells are scarce. Indeed, differences in the median number of EpCAM+ cells in morphological positive or negative MPE reveal that FCI can discover MPE with EpCAM+ cells in the range of 0.01%. Using a different technology, Thompson et. al reported similar findings in PE samples from NSCLC patients [26].
From a practical point of view, FCI worked as a screening test and early detection of FCI + cases accelerated the diagnostic pathway of unsuspected MPE. On the other side, high-sensitivity FCI may detect EpCAM+ cells in specific clinical situations not associated with malignancy. The cell-adhesion molecule EpCAM describes epithelial cells, but the category of “malignant epithelial cells” depends on where they are detected. Serous cavity fluids lack epithelial cells, but disruption of epithelial integrity as seen in recent surgeries [9], inflammatory diseases, pneumonia, tuberculosis [10] or pericardial drainage, might release normal airway epithelial cells into PE. Benign epithelial cells have also been described in peritoneal washings from patients with advanced cirrhosis [11] and this might be the case of our patient diagnosed with an alcoholic hepatitis. Indolent clonal B-cell populations without clinical relevance can also be found in PE. These low percentages of clonal lymphocytes may be difficult to distinguish from MPE minimally infiltrated by low-grade NHL. An extended FCI study should help because clonal B-cells with immunophenotype of chronic lymphocytic leukemia or marginal-zone lymphoma may develop in patients with no need of further therapy, while identification of follicular lymphoma supports MPE.
Clinicians play an important role in the success of this approach because they choose which FCI study should be performed. Samples initially triaged to discard epithelial cell neoplasms and finally diagnosed with high-grade lymphoma explain some of our FCI-/Cy + results. High sensitive FCI relies on the analysis of a very high number of cells, so no sample will be left to expand a lymphocytic study if finally needed. Some authors prefer to evaluate both malignancies in a single tube [10], but we separated them based on the low frequency of MPE secondary to lymphoma and the predominance of lymphocytes in many reactive PE [3, 27, 28]. Furthermore, the use of a 3-color epithelial tube [5, 7] while keeping costs low, also helps limiting background interference.
Using a single target of epithelial cells implies that tumors with weak or absent EpCAM expression, such as poorly differentiated tumors or epithelial-mesenchymal transition [29], might escape FCI detection. To improve the efficiency to detect frequent EpCAM-negative tumors such as SSC [30], FCI might include reagents directed against other epithelial cell antigens [31]. As previously described [4, 10] including CD56 (neural cell adhesion molecule) facilitates the identification of neuroendocrine tumors, and potential therapeutic targets, such as that recently described for PDL-1, could also be considered for the next FCI panels [32]. A recent FCI report includes a description of a design for an 8-color antibody panel to identify pediatric solid tumors [33]. Combining similar strategies with the high-sensitivity FCI approach would not only help to detect MPE, but also to locate its primary origin.
Conclusions
Clinical implementation of high sensitivity FCI as part of the diagnostic work-up of PE with suspicion of malignancy significantly increases the diagnostic yield of MPE. Even clinical laboratories with limited resources can easily perform the 3-color epithelial cell panel. By using FCI as a screening technique, implies that the early detection of FCI + cases accelerates the diagnostic pathway of unsuspected MPE.
Abbreviations
- MPE:
-
Malignant pleural effusions
- PE:
-
Pleural effusions
- FCI:
-
Flow cytometry immunophenotyping
- EpCAM:
-
Epithelial cell adhesion molecule
- PET:
-
Positron emission tomography
- mAb:
-
Monoclonal antibodies
- FITC:
-
Fluorescein isothiocyanate
- PerCP:
-
Peridinin Chlorophyll Protein Complex
- APC:
-
Allophycocyanin
- EpCAM:
-
Epithelial cell adhesion molecule
- LOD:
-
Limit of detection
- LLOQ:
-
Lower limit of quantification
- IQR:
-
Interquartile range
- M:
-
Male
- F:
-
Female
- PEL:
-
Primary effusion lymphoma
- NHL:
-
Non-Hodgkin lymphoma
- SSC:
-
Squamous cell carcinoma
- NSCLC:
-
Non-small cell lung cancer
- NET:
-
Neuroendocrine tumor
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
References
Arnold DT, Fonseka D, De, Perry S, Morley A, Harvey JE, Medford A, Brett M, Maskell NA (2018) Investigating unilateral pleural effusions: the role of cytology. Eur Respir J 52:1–9. https://doi.org/10.1183/13993003.01254-2018
Zhang M, Yan L, Lippi G, Hu Z, De (2021) Pleural biomarkers in diagnostics of malignant pleural effusion: a narrative review. Translational Lung Cancer Research 10:1557–1570. https://doi.org/10.21037/tlcr-20-1111
Dixon G, Bhatnagar R, Zahan-Evans N, Clive AO, Virgo PF, Brett MT, Otton SH, Medford ARL, Maskell NA (2018) A prospective study to evaluate a diagnostic algorithm for the use of fluid lymphocyte subset analysis in undiagnosed unilateral pleural effusions. Respiration 95:98–105. https://doi.org/10.1159/000481290
Chang A, Benda PM, Wood BL, Kussick SJ (2003) Lineage-specific identification of nonhematopoietic neoplasms by flow cytometry. Am J Clin Pathol 119:643–655. https://doi.org/10.1309/FU3FDKYN8AU0891N
Acosta M, Pereira J, Arroz M (2016) Screening of carcinoma metastasis by flow cytometry: a study of 238 cases. Cytometry part B -. Clin Cytometry 90:289–294. https://doi.org/10.1002/cyto.b.21258
Wong-Arteta J, Rey M, Aragón L, Gil-Rodríguez E, Bujanda L (2020) The utility of flow cytometry in the diagnostic work up of malignant effusions due to nonhematopoietic neoplasms. Cytometry part B -. Clin Cytometry 98:504–515. https://doi.org/10.1002/cyto.b.21886
Pillai V, Cibas ES, Dorfman DM (2013) A simplified flow cytometric immunophenotyping procedure for the diagnosis of effusions caused by epithelial malignancies. Am J Clin Pathol 139:672–681. https://doi.org/10.1309/AJCP4HIFSHMO9WTK
Kentrou NA, Tsagarakis NJ, Tzanetou K, Damala M, Papadimitriou KA, Skoumi D, Stratigaki A, Anagnostopoulos NI, Malamou-Lada E, Athanassiadou P, Paterakis G (2011) An improved flow cytometric assay for detection and discrimination between malignant cells and atypical mesothelial cells, in serous cavity effusions. Cytometry Part B - Clinical Cytometry 80 B:324–334. https://doi.org/10.1002/cyto.b.20608
Sahu S, Gupta P, Susheilia S, Gautam U, Dey P (2021) Application of multicolour flow cytometry in the detection of metastatic carcinoma in serous effusions: special emphasis in atypical cytology. Cytopathology 32:169–179. https://doi.org/10.1111/cyt.12922
Quirós-Caso C, Arias Fernández T, Fonseca-Mourelle A, Torres H, Fernández L, Moreno-Rodríguez M, Ariza-Prota MÁ, López-González FJ, Carvajal-Álvarez M, Alonso-Álvarez S, Moro-García MA, Colado E (2022) Routine flow cytometry approach for the evaluation of solid tumor neoplasms and immune cells in minimally invasive samples. Cytometry Part B - Clinical Cytometry 1–11. https://doi.org/10.1002/cyto.b.22081
Risberg B, Davidson B, Dong HP, Nesland JM, Berner A (2000) Flow cytometric immunophenotyping of serous effusions and peritoneal washings: comparison with immunocytochemistry and morphological findings. J Clin Pathol 53:513–517. https://doi.org/10.1136/jcp.53.7.513
Hooper C, Lee YCG, Maskell N, Pleural BTS (2010) Investigation of a unilateral pleural effusion in adults : british thoracic society pleural disease guideline 2010. 65. https://doi.org/10.1136/thx.2010.136978
Pinto D, Chandra A, Crothers BA, Kurtycz DFI, Schmitt F (2020) The international system for reporting serous fluid cytopathology—diagnostic categories and clinical management. J Am Soc Cytopathol 9:469–477. https://doi.org/10.1016/j.jasc.2020.05.015
Samper GJ, Herrera S, Estrella L, Marco J, Rafael B, Lapiedra A, Pinilla A (2017) Predicting Malignant and Paramalignant Pleural Effusions by combining Clinical, Radiological and Pleural Fluid Analytical Parameters. 653–660. https://doi.org/10.1007/s00408-017-0032-3
van Dongen JJM, Lhermitte L, Böttcher S, van der Almeida J, Flores-Montero J, Rawstron A, Asnafi V, Lécrevisse Q, Lucio P, Mejstrikova E, Szczepański T, Kalina T, de Tute R, Brüggemann M, Sedek L, Cullen M, Langerak AW, Mendonça A, Macintyre E, Martin-Ayuso M, Hrusak O, Vidriales MB, Orfao A (2012) EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia 26:1908–1975. https://doi.org/10.1038/leu.2012.120
Orfao A, Matarraz S, Pérez-Andrés M, Almeida J, Teodosio C, Berkowska MA, van Dongen JJM (2019) Immunophenotypic dissection of normal hematopoiesis. J Immunol Methods 475:112684. https://doi.org/10.1016/j.jim.2019.112684
Arroz M, Came N, Lin P, Chen W, Yuan C, Lagoo A, Monreal M, de Tute R, Vergilio JA, Rawstron AC, Paiva B (2016) Consensus guidelines on plasma cell myeloma minimal residual disease analysis and reporting. Cytometry part B -. Clin Cytometry 90:31–39. https://doi.org/10.1002/cyto.b.21228
Pillai V, Dorfman DM (2016) Flow cytometry of nonhematopoietic neoplasms. Acta Cytol 60:336–343. https://doi.org/10.1159/000448371
Husain AN, Colby TV, Ordóñez NG, Allen TC, Attanoos RL, Beasley MB, Butnor KJ, Chirieac LR, Churg AM, Dacic S, Galateau-Sallé F, Gibbs A, Gown AM, Krausz T, Litzky LA, Marchevsky A, Nicholson AG, Roggli VL, Sharma AK, Travis WD, Walts AE, Wick MR (2018) Guidelines for pathologic diagnosis of malignant mesothelioma: 2017 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 142:89–108. https://doi.org/10.5858/arpa.2017-0124-RA
del Díaz C, Saiz-Pardo Sanz M, Ortega Medina L, Fernández Aceñero MJ (2021) Ber-EP4 staining in effusion cytology: a potential source of false positives. Revista Esp de Patologia 54:114–122. https://doi.org/10.1016/j.patol.2020.04.005
Attanoos RL, Griffin A, Gibbs AR (2003) The use of immunohistochemistry in distinguishing reactive from neoplastic mesothelium. A novel use for desmin and comparative evaluation with epithelial membrane antigen, p53, platelet-derived growth factor-receptor, P-glycoprotein and Bcl-2. Histopathology 43:231–238. https://doi.org/10.1046/j.1365-2559.2003.01686.x
Maguire B, Whitaker D, Carrello S, Spagnolo D (1994) Monoclonal antibody Ber-EP4: its use in the differential diagnosis of malignant mesothelioma and carcinoma in cell blocks of malignant effusions and FNA specimens. Diagn Cytopathol 10:130–134
Salisbury T, Churg A (2021) CD146 immunohistochemical staining for the separation of benign from malignant mesothelial proliferations. Virchows Arch 479:1047–1050
Sato A, Torii I, Okamura Y, Yamamoto T, Nishigami T, Kataoka TR, Song M, Hasegawa S, Nakano T, Kamei T, Tsujimura T (2010) Immunocytochemistry of CD146 is useful to discriminate between malignant pleural mesothelioma and reactive mesothelium. Mod Pathol 23:1458–1466. https://doi.org/10.1038/modpathol.2010.134
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, Munshi N, Lonial S, Bladé J, Mateos MV, Dimopoulos M, Kastritis E, Boccadoro M, Orlowski R, Goldschmidt H, Spencer A, Hou J, Chng WJ, Usmani SZ, Zamagni E, Shimizu K, Jagannath S, Johnsen HE, Terpos E, Reiman A, Kyle RA, Sonneveld P, Richardson PG, McCarthy P, Ludwig H, Chen W, Cavo M, Harousseau JL, Lentzsch S, Hillengass J, Palumbo A, Orfao A, Rajkumar SV, Miguel JS, Avet-Loiseau H (2016) International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 17:e328–e346. https://doi.org/10.1016/S1470-2045(16)30206-6
Thompson JC, Fan R, Black T, Yu GH, Savitch SL, Chien A, Yee SS, Sen M, Hwang WT, Katz SI, Feldman M, Vachani A, Carpenter EL (2019) Measurement and immunophenotyping of pleural fluid EpCAM-positive cells and clusters for the management of non-small cell lung cancer patients. Lung Cancer 127:25–33. https://doi.org/10.1016/j.lungcan.2018.11.020
Fuller MY, Thrall MJ (2016) Utility of flow cytometry analysis for pleural and peritoneal fluids. J Am Soc Cytopathol 5:339–344. https://doi.org/10.1016/j.jasc.2016.07.001
Plummer RM, Kelting S, Madan R, O’Neil M, Dennis K, Fan F (2020) Triaging of pleural effusion cytology specimens for ancillary flow cytometric analysis. J Am Soc Cytopathol 9:478–484. https://doi.org/10.1016/j.jasc.2020.07.131
Jie XX, Zhang XY, Xu CJ (2017) Epithelial-to-mesenchymal transition, circulating tumor cells and cancer metastasis: mechanisms and clinical applications. Oncotarget 8:81558–81571. https://doi.org/10.18632/oncotarget.18277
Spizzo G, Fong D, Wurm M, Ensinger C, Obrist P, Hofer C, Mazzoleni G, Gastl G, Went P (2011) EpCAM expression in primary tumour tissues and metastases: an immunohistochemical analysis. J Clin Pathol 64:415–420. https://doi.org/10.1136/jcp.2011.090274
Davidson B, Dong HP, Berner A, Christensen J, Nielsen S, Johansen P, Bryne M, Asschenfeldt P, Risberg B (2002) Detection of malignant epithelial cells in effusions using flow cytometric immunophenotyping: an analysis of 92 cases. Am J Clin Pathol 118:85–92. https://doi.org/10.1309/M877-QABM-D9GB-FJAX
Yoon JY, Nayyar R, Quest G, Pabedinskas D, Pal P, Tsao MS, Schwock J, Ko HM (2020) PD-L1 lineage-specific quantification in malignant pleural effusions of lung adenocarcinoma by flow cytometry. Lung Cancer 148:55–61. https://doi.org/10.1016/j.lungcan.2020.07.013
De Sá Ferreira-Facio C, Botafogo V, Ferrão PM, Canellas MC, Milito CB, Romano S, Lopes DV, Teixeira LC, Oliveira E, Bruno-Riscarolli E, Mello FV, Siqueira PFR, Moura P, Macedo FN, Forny DN, Simião L, Pureza AL, Poirot Land MG, Pedreira CE, Van Dongen JJM, Orfao A, Da Costa ES (2021) Flow cytometry immunophenotyping for diagnostic orientation and classification of pediatric cancer based on the euroflow solid tumor orientation tube (stot). Cancers 13. https://doi.org/10.3390/cancers13194945
Acknowledgements
The authors greatly acknowledge support by Lawrence JC Baron for editing the manuscript.
Author information
Authors and Affiliations
Contributions
Dolores Subirá designed and conducted the research, analyzed and interpreted data, wrote the manuscript, and critically revised it. Jesús Fernández, Jorge Castelao and Luis Chara were responsible for clinical supervision. Fabiola Barriopedro and Ruth Martínez provided technical assistance and Fabiola Barriopedro collected data. Eugenia García supervised pathological data. The manuscript was written by Dolores Subirá. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Declarations
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. The authors have no relevant financial or non-financial interests to disclose.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Subirá, D., Barriopedro, F., Fernández, J. et al. High sensitivity flow cytometry immunophenotyping increases the diagnostic yield of malignant pleural effusions. Clin Exp Metastasis 40, 505–515 (2023). https://doi.org/10.1007/s10585-023-10236-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-023-10236-4