Abstract
Lower limb pathological fractures caused by bone metastases can severely impair activities of daily living, so recognizing fracture risk is essential. Medial cortical involvement (MCI) in the proximal femur has been demonstrated to affect bone strength in biomechanical studies, but it has not been investigated in real patients. Between 2012 and 2019, 161 bone metastases with computed tomography (CT) images were retrospectively examined. Twenty-nine fractures were observed including 14 metastases with pathological fractures at the first examination, and prophylactic surgery was performed for 50 metastases. We extracted clinicopathological data using CT images, including patient’s background, MCI in the proximal femur, site, size, circumferential cortical involvement (CCI), pain, and nature of metastasis. Cox proportional hazard regression analyses were performed, and we created integer scores for predicting fractures. We revealed that MCI, CCI, lytic dominant lesion, and pain were significant factors by univariate analyses. By multivariable analysis, MCI and each 25% CCI were significant and integer score 1 was assigned based on hazard ratio. The full score was four points, with MCI in the proximal femur (one point) and ≥ 75% CCI (three points). With integer score two, sensitivity was 88.9% and specificity was 81.2% for predicting fracture within 60 days. In conclusion, MCI and CCI examined by CT images were the risk factors for pathological fracture. CCI ≥ 50% is a widely known risk factor, but in addition, it may be better to consider surgery if MCI in the proximal femur is observed in metastasis with 25–50% CCI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of molecular-targeted drugs and immune-checkpoint inhibitors has contributed to better survival rates in advanced-stage cancers [1, 2]. Bone is the third most common site of metastasis, after the lungs and the liver [3, 4], and avoiding skeletal related events (SREs) is important for maintaining activities of daily living (ADL) and quality of life (QOL) [5, 6]. Particularly, lower limb fractures can severely impair ADL and QOL [5, 6]. In addition, prophylactic surgery for femur yielded better results than surgery for completed fractures with shorter hospital stay, greater likelihood of discharge to home, and greater walking ability [7].
To avoid pathological fractures, recognition of high-risk metastatic lesions is essential, while on the other hand, patients with a low risk of pathologic fractures should be spared overtreatment. Therefore, there have been many studies predicting pathological fractures. A representative study was performed by Mirels in 1989 [8], which reported site, nature, size, and pain as the prognostic factors for predicting fractures. Van der Linden reported cortical involvement is important in 2003 [9]. These factors were widely known and used for predicting pathological fractures.
There are several areas for improvement with regards predicting pathological fractures. First, medial cortical involvement (MCI) in the proximal femur has not been assessed, which is important for bone strength certified by biomechanical analysis [10,11,12,13,14,15]. Second, there have been fewer studies in recent years, despite the treatments for cancer having made much progress, including the introduction of bone modifying agents (BMA). Third, patients with a fracture at the first presentation, or patients with impending fracture who would receive surgery soon after the first presentation, might not be included into prospective analyses due to ethical problems in recent years. Forth, regarding the nature of the metastases, intertrabecular metastases have not been analyzed, and mixed lesions represent too wide a range of metastasis. Finally, many studies did not use computed tomography (CT) images for evaluating the pathological fracture risk, although it is routinely used for detecting systemic metastases, and is recommended for measuring the value of axial cortical involvement and other risk factors to further improve fracture risk prediction [16].
The purpose of this study is the re-evaluation of risk factors of pathological fracture in lower limb metastases. We included metastases with fracture or impending fracture, and investigated fracture risk by Cox proportional hazard regression analysis for considering time course. In addition to Mirels’ classification, we explored factors associated with site, size, nature, and pain in detail and compared to Mirels’ classification using only CT images. Then we aimed to construct a new scoring system for predicting pathological fractures.
Patients and methods
Study design
This retrospective study was conducted at two university hospitals and one cancer center in Japan.
Participants and data collection
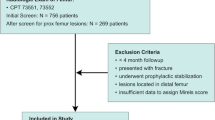
The records of patients with lower limb bone metastasis who were consulted to an orthopedic surgeon in the three institutions between April 2012 and March 2019, were included in the study. In one university hospital and one cancer center, mainly patients with fracture, impending fracture, or pain were consulted to orthopedic surgeons. Meanwhile, in the other university hospital, which has a multidisciplinary team for the treatment of bone metastases, patient was consulted to an orthopaedic surgeon when bone metastasis was detected, even if fracture risk was low. Although the timing of consultation to orthopaedic surgeons were different, the treatment strategy and surgical indication for lower limb metastases in three institutions were same as shown in Fig. 1. Bone metastases were detected using CT, magnetic resonance imaging (MRI), Tc-99 m MDP bone scintigraphy, or fluorodeoxyglucose-positron emission tomography (FDG-PET). Ultrasound or CT-guided percutaneous biopsy was performed in cases of inconclusive lesions. Only bone metastases with CT images were included in this study. We also included patients who had a pathological fracture in the lower limb at the first examination.
We defined the day of CT scan covering lower limb metastasis as day zero. Then we defined the final follow-up date; for patients with fracture: the fracture date; for patients who underwent prophylactic surgery: the date of surgery; and for patients without fracture or surgery: the final follow-up date. For patients with fracture at first examination, the follow-up period was defined as zero (Supplementary Table 1).
We extracted the following clinicopathological data on day zero: age, sex, primary lesion, history of chemotherapy, history of BMA, history of local radiation therapy, laboratory data (serum C-reactive protein [CRP], and albumin [Alb]), and whether patients had pain (categorized to functional pain on weight-bearing, and other pain) caused by the metastasis. Primary lesions of the metastases were classified based on Katagiri’s score [17]. Lung cancer was classified into two groups based on the presence of the Epidermal Growth Factor Receptor [EGFR] mutation or anaplastic lymphoma kinase [ALK] rearrangement. Prostate cancers were classified into hormone-sensitive and hormone-resistant groups. Breast cancers were classified into hormone-receptor positive and negative groups. We then examined whether patients had BMA administration, local radiation therapy, or surgery for lower limb metastasis during the follow-up period.
Next, we examined the metastasis using axial CT images and extracted the following data: site (peri-trochanteric lesion, or other lower limb skeleton), MCI in the proximal femur (yes or no), size according to Mirels’ score (tumor/bone width < 1/3, 1/3–2/3, and > 2/3), CCI (< 25%, < 50%, < 75%, and ≥ 75%), and nature (five-tiered approach: intertrabecular, lytic, mixed [lytic dominant or blastic dominant], and blastic). We defined the proximal femur as the femoral head, femoral neck, intertrochanteric lesion, and femoral shaft proximal to the lower edge of the lesser trochanter. The medial cortex at the proximal femur was defined as thickened cortical bone at the medial neck or the antero-medial quarter of the femoral section anterior to the lesser trochanter (Fig. 2) [10, 11]. Cortical involvement was defined as any detectable change in the cortical bone by axial CT images. Intertrabecular metastasis was defined as those which made no detectable change in the cortical bone on CT images. With regards to nature, we made a two-tiered classification, which divided the nature into lytic dominant lesion (lytic and lytic dominant lesions in five-tiered classification) and others (blastic, blastic dominant, and intertrabecular). For Mirels’ classification of nature, we categorized intertrabecular metastases into blastic lesion.
Analysis of fracture risk
The fracture risk was investigated using Cox proportional hazard regression analysis. We analyzed fracture risk by the patient’s background and factors related to the metastasis itself. Site was classified by Mirels’ classification or the existence of MCI in the proximal femur. Size was classified by Mirels’ classification or CCI. Nature was classified by five-tiered, Mirels’, or two-tiered classification. Pain was classified by Mirels’ classification or the presence of pain (yes or no) including moderate and functional pain in Mirels’ classification. Definition of event occurrence and censoring was shown in Supplementary Fig. 1. A p-value < 0.05 was regarded as statistically significant. Factors referred to the metastasis itself, with higher hazard ratio and lower p-value, were investigated by multivariable Cox proportional hazard regression analysis. The integer score was calculated by hazard ratio.
A survival curve assessing fracture risk was drawn by integer score using Kaplan–Meier method, and a Log-rank test was performed with reference to score 0–1. A receiver operating characteristic (ROC) curve was drawn using logistic regression analysis and area under the curve (AUC) was calculated. Inclusion and categorization of data was shown in Supplementary Table 2.
Statistical analysis
Statistical analyses were performed using JMP® 14 (SAS Institute Inc., Cary, NC, USA).
Results
Between April 2012 and March 2019, the number of patients with lower limb bone metastasis in our institutions was 172, and among them, local CT images were available in 154 patients, with 161 lower limb bone metastases.
Table 1 shows the baseline characteristics of the patients. Mean age was 65.7 years, and 50% of the patients were male. With regards to the primary lesion of the bone metastases, lung cancer was the most common, followed by breast cancer and renal cell carcinoma. According to Katagiri’s classification, 44.2% of the primary lesions were rapid growth tumors. BMA were used in 13 (8.4%) patients, and started administration during follow-up in 50 (32.5%) patients. Mean serum CRP was 3.16 mg/dL and Alb was 3.55 g/dL.
Supplementary Table 1 shows the frequency of the fracture and the follow-up period. Among the 161 metastases, fracture occurred in 29 metastases, including 14 metastases which had already fractured and were identified at the first examination. Fifteen patients fractured during follow-up with median interval of nine days (Interquartile range, IQR 4–40). All fractured patients underwent surgery for metastases. We performed prophylactic surgery for 50 metastases. Median follow-up period for metastases without fracture was 42.5 days (IQR 13–132). Median follow-up period across all patients was 33.0 days (IQR 10–115).
Table 2 shows the fracture risk based on Cox proportional hazard regression analysis. BMA administration, MCI at proximal femur, size, nature, and pain were significantly related to fracture risk.
With regards BMA, no fracture occurred in patients with BMA induction before CT evaluation. Patients with administration of BMA during follow-up had a significantly lower risk of fracture.
With respect to tumor size, metastases with larger ratio of tumor size/bone width had significantly higher risk with reference to size < 1/3. CCI was significantly related to fracture risk and hazard ratio (HR) increased as the involvement area increased.
Regarding the nature of the metastasis, fracture risk was significantly higher only on lytic metastasis in five-tiered classification (HR, 7.73; p = 0.048) with reference to intertrabecular metastases. Fracture risk on blastic metastasis was not significantly lower compared to that on intertrabecular metastasis. In Mirels’ classification, mixed and lytic lesions had significantly higher risk of fracture (HR, 2.22 and 3.24, respectively). Fracture risk was significantly high in lytic dominant lesions (HR, 5.83; p = 0.004) by two-tiered classification.
When pain was classified according to Mirels’ classification, metastases with moderate pain (HR, 6.23; p = 0.0004) and functional pain (HR, 3.19; p = 0.04) had higher risk compared to those with mild pain, but the hazard ratio was not increased as the score increased. Meanwhile, metastases with any pain had higher fracture risk (HR, 4.72; p = 0.002) compared to those without pain.
Table 3 shows the results of the multivariable analysis of fracture risk. MCI at the proximal femur (HR, 2.35; p = 0.03) and CCI (unit HR, 3.13; p < 0.0001) were significantly related to fracture. We assigned the integer score 1 to MCI in the proximal femur and each 25% CCI. The full score was four points, with MCI in the proximal femur (one point) and ≥ 75% CCI (three points). The number of patients with integer score 0 point was 70, 1 point: 32, 2 points:18, 3 points:26, and 4 points:15.
Figure 3 shows the survival curve assessing fracture risk with prognostic scores calculated by the integer score by Kaplan–Meier method. The survival rate of score 2, 3, and 4 was each significantly different from that of score 0–1.
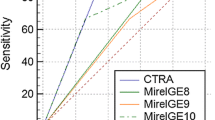
Figure 4a shows the ROC curve created based on metastases with fracture within 60 days and those without fracture over 60 days (n = 114). The AUC was 0.924 for new prognostic score and 0.827 for Mirels’ score. With integer score 2, sensitivity was 88.9% and specificity was 81.2%. Figure 4b shows the ROC curve created by metastases excluding those with fractures at the first examination. The AUC was 0.878 for the new score and 0.843 for Mirels’ score.
Table 4 shows the fracture risk by prognostic score. The Fracture risk in 60 days was 8.0% with a score of 1, 28.0% with a score of 2, 63.4% with a score of 3, and 88.6% with a score of 4.
Discussion
We retrospectively analyzed the risk factors of pathological fractures in lower limb metastases using CT images. Twenty-nine metastases fractured among 161 metastases, and BMA administration, MCI at the proximal femur, CCI, lytic dominant lesions (two-tiered classification), and presence of pain were significantly related to pathological fracture by univariate Cox proportional hazard regression analysis. In the multivariable analysis, among factors related to the local lesion, MCI and 25% each CCI was significantly related. We constructed a scoring system to predict pathological fractures by assigning an integer score 1 to both. A Full score was four points with ≥ 75% CCI, and MCI in the proximal femur. We plotted a ROC curve investigating 60-days fracture risk and the AUC was 0.924. With integer score 2, sensitivity was 88.9% and specificity was 81.2% for pathological fractures.
In this study, we included metastases with fractures or impending fractures which might have the highest risk of fracture. For the last two decades, there have been studies analyzing the fracture risk after radiation therapy, but it might be possible these studies did not include metastases at high risk. In many institutions in Japan, prophylactic surgery may treat impending fractures, soon after the first examination and these metastases might not be included in these studies. As the analysis of high-risk metastases are important for investigating the risk factors of fracture, we included metastases with fracture and impending fracture, and analyzed by Cox proportional hazard regression methods taking time course into account. Meanwhile, we also included many patients with metastases at low risk of fracture who were consulted to a multidisciplinary team soon after the diagnosis of bone metastasis. As a result, we included metastases with a wide range of fracture risks (Fig. 3).
In the present study, we focused on MCI in the proximal femur, and showed that any detectable change to the lesion on CT images increased the risk of fracture. Keyak et. al. first described defects of the medial cortex highly affecting the bone strength using finite element (FE) methods [10]. Many studies have shown the defect at the lesion was the risk factor for pathological fracture using FE methods or biomechanical analyses [11,12,13,14,15]. However, there has been no study which examined the risk of MCI in the proximal femur compared with other risk factors. This is the first study to demonstrate that involvement of the lesion was a significant risk factor in multivariable analysis.
With regards the nature of the metastases, many studies have analyzed the risk by classifying metastases into lytic, blastic, and mixed lesions. However, in reality, there are many intertrabecular metastases [18, 19]. We defined intertrabecular metastases as those which made no detectable change in the cortical bone on CT images. In addition, conventional mixed lesions have a very wide range of images, thus, we divided the mixed lesions into lytic dominant and blastic dominant. As a result, we constructed a five-tiered classification including intertrabecular metastases. As there has been no study investigating the mechanical strength of intertrabecular metastases, we analyzed the risk of fracture, and showed the fracture risk of intertrabecular metastases might be low compared with that of other metastases, although not significant. Our results showed the hazard ratio of 5-tiered classification was increased as the lytic factor increased, although not significantly so due to the small sample size. This showed the potential usefulness of this classification. We then constructed a 2-tiered classification: lytic dominant (lytic and lytic dominant in 5-tiered classification), and others (blastic dominant, blastic, and intertrabecular type). In the present study, this classification was of most use when predicting the fracture risk. In this classification, the hazard ratio for lytic dominant lesions was 5.83 with a p-value < 0.004, but it was not significant in the multivariable analysis.
Pain is reported as a risk factor of pathological fracture. Mirels classified the pain into mild, moderate, and functional pain, but our results showed the hazard ratio of moderate pain was higher than that of functional pain. Thus, we classified the pain into two groups: metastases with pain, or without pain. This classification is quite simple and useful, however it was not significant in the multivariable analysis. Although several studies reported increased pain was the risk factor [20], and prophylactic internal fixation was recommended in cases of increasing pain [21], we could not examine this because our study was retrospective. Pain has been shown to be related to fracture to some extent, but despite patients not complaining of pain, fracture might occur. We showed that early detection of bone metastasis from hepatocellular carcinoma before the symptoms arise might lead to prevention of pathological fracture or paralysis [22]. Therefore, caution to not miss asymptomatic metastases in patient should be taken, particularly with impending fracture or paralysis, when consulted by the oncologist.
The most influential risk factor of pathological fracture reported is cortical involvement. The oldest study found on this topic was published in 1981 [23]. Based on plain radiographs, the author estimated the percentage of metastatic circumferential involvement by dividing them roughly into four categories of cortex involved: less than 25%, 25–50%, 50–75%, and over 75%. It was concluded that metastatic long bones involving over 50% of the cortex were likely to fracture and should be considered for prophylactic fixation. Cortical destruction or involvement ≥ 50% has been reported as a risk factor in other studies using plain radiographs [9, 20, 24]. However, error ranges may be wide for predicting the cortical involvement from plain radiographs, thus we used CT as our imaging modality. There was a report, using CT images, that cortical involvement ≥ 30% was a risk factor [25]. In the present study, fracture risk at 60 days with integer score 2 was 28.0% (Table 4), which was considered high risk. Therefore, if cortical involvement is 25–50% with involvement of the medial cortex in the proximal femur, or if cortical involvement is over 50%, surgery may have to be considered, regardless of pain or nature.
In the present study, excluding patients with fracture at the first examination, we performed surgery on 12.7% of zero points, 48.4% of one point, and 81.1% of 2–4 points metastases (data not shown). It is imperative to avoid overtreatment for metastases, because surgical overtreatment also unnecessarily increases morbidity (e.g., hospitalization, general anesthetics, and complications arising from a forced supine position) in patients whose life expectancy is already limited [25]. However, the reason we performed surgery for lower risk metastases might be the sensitivity to radiation therapy as shown in our strategy (Fig. 1). If the sensitivity to radiation and systemic therapy was low, metastases might increase and involve the cortex soon, which leads to fracture. Therefore, in addition to this scoring system, we should consider increasing speed and sensitivity for systemic therapy or radiation therapy for deciding surgical indications.
By univariate analysis, BMA administration was significantly related to fractures among systemic factors. We included BMA into the analyses because BMA has been reported to reduce fracture risk in many studies. However, we did not include BMA in the multivariate analysis in the present study, because the follow-up period was too short to see the effect, and BMA administration may have a large selection bias. As shown in Fig. 1, if the metastasis is fractured or at high risk, we perform surgery as soon as possible, and generally we administrate BMA after surgery. On the other hand, if we decide not to perform surgery, we will initiate BMA. Therefore, in the follow-up period in this study, we used BMA only on patients with medium or low risk of fracture, which created the selection bias. When we start BMA, we have recognized the metastases and taken care not to fracture. We perform prophylactic surgery if needed, which leads to a low rate of fracture occurrence in the BMA administrated group, in retrospective studies such as this. In the present study, indeed, no fracture occurred in 14 patients who had been administered BMA before the follow-up period. In other words, detecting metastases at low or medium risk may be important for preventing fractures.
In the same way, radiation therapy may have large selection bias in this study. We gave priority to surgery when the metastasis was at high risk of fracture, because radiation therapy is proven not to be significantly effective for preventing fracture [26]. We only performed radiation therapy to metastases at medium risk, or in the case that we had to wait for surgery for metastases at high risk for various reasons.
The present study has some limitations. First, this is a retrospective study, and included metastases with fractures at the first presentation for analyzing metastases with higher risk. When we examined CT images after fracture, we could not measure the exact size of the tumor or exact range of cortical involvement. However, we could not perform a prospective study because we cannot wait and see the impending fracture till fracture occurs. In addition, some risk factors for fracture could not be included, such as assistant devices, weight-bearing status, bone density status, smoking status, and osteoporosis comorbidity in addition to BMA administration and local radiation therapy. Second, we did not confirm the pathology of metastases.
In conclusion, MCI in the proximal femur and CCI examined by CT images were the risk factors for pathological fracture. CCI ≥ 50% is a widely known risk factor, but in addition, it may be better to consider surgery if the MCI in the proximal femur is observed in metastasis with 25–50% CCI.
Abbreviations
- SREs:
-
Skeletal related events
- ADL:
-
Activities of daily living
- QOL:
-
Quality of life
- MCI:
-
Medial cortical involvement
- BMA:
-
Bone-modifying agent
- CT:
-
Computed tomography
- CRP:
-
C reactive protein
- Alb:
-
Albumin
- CCI:
-
Circumferential cortical involvement
- ROC:
-
Receiver Operating Characteristic
- AUC:
-
Area under the curve
- IQR:
-
Interquartile range
- HR:
-
Hazard ratio
- FE method:
-
Finite element method
References
Maemondo M, Inoue A, Kobayashi K et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–2388
Robert C, Long GV, Brady B et al (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372:320–330
Hage WD, Aboulafia AJ, Aboulafia DM (2000) Incidence, location, and diagnostic evaluation of metastatic bone disease. Orthop Clin N Am 31:515–528
Hosono N, Yonenobu K, Fuji T, Ebara S, Yamashita K, Ono K (1995) Orthopaedic management of spinal metastases. Clin Orthop Relat Res 312:148–159
Coleman RE (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27:165–167
Shinoda Y, Sawada R, Yoshikawa F et al (2019) Factors related to the quality of life in patients with bone metastases. Clin Exp Metastasis 36:441–448
Ward WG, Holsenbeck S, Dorey FJ, Spang J, Howe D (2003) Metastatic disease of the femur: surgical treatment. Clin Orthop Relat Res 415S:S230–244
Mirels H (1989) Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res 249:256–264
Van der Linden YM, Kroon HM, Dijkstra SP et al (2003) Simple radiographic parameter predicts fracturing in metastatic femoral bone lesions: results from a randomized trial. Radiother Oncol 69:21–31
Keyak JH, Kaneko TS, Skinner HB et al (2007) The effect of simulated metastatic lytic lesions on proximal femoral strength. Clin Orthop Relat Res 459:139–145
Shinoda Y, Kobayashi H, Kaneko M et al (2019) Prediction of the pathological fracture risk during stance and fall-loading configurations for metastases in the proximal femur, using a computed tomography-based finite element method. J Orthop Sci 24:1074–1080
Kawabata Y, Matsuo K, Nezu Y et al (2017) The risk assessment of pathological fracture in the proximal femur using a CT-based finite element method. J Orthop Sci 22:931–937
Sivasundaram R, Shah S, Ahmadi S et al (2013) The biomechanical effect of proximal tumor defect location on femur pathological fractures. J Orthop Trauma 27:e174–e180
Benca E, Reisinger A, Patsch JM et al (2017) Effect of simulated metastatic lesions on the biomechanical behavior of the proximal femur. J Orthop Res 35:2407–2414
Tanck E, van Aken JB, van der Linden YM et al (2009) Pathological fracture prediction in patients with metastatic lesions can be improved with quantitative computed tomography based computer models. Bone 45:777–783
Hamaoka T, Madewell JE, Podoloff DA et al (2004) Bone imaging in metastatic breast cancer. J Clin Oncol 22:2942–2953
Katagiri H, Okada R, Takagi T et al (2014) New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med 3:1359–1367
Yamaguchi T, Tamai K, Yamato M et al (1996) Intertrabecular pattern of tumors metastatic to bone. Cancer 78:1388–1394
Yamaguchi T (2001) Intertrabecular vertebral metastases: metastases only detectable on MR imaging. Semin Musculoskelet Radiol 5:171–175
Harrington KD (1982) New trends in the management of lower extremity metastases. Clin Orthop Relat Res 169:53–61
Dijkstra PD, Oudkerk M, Wiggers T (1997) Prediction of pathological subtrochanteric fractures due to metastatic lesions. Arch Orthop Trauma Surg 116:221–224
Hirai T, Shinoda Y, Tateishi R (2019) Early detection of bone metastases of hepatocellular carcinoma reduces bone fracture and paralysis. Jpn J Clin Oncol 49:529–536
Fidler M (1981) Incidence of fracture through metastases in long bones. Acta Orthop 52:623–627
Menck H, Schulze S, Larsen E (1988) Metastasis size in pathologic femoral fractures. Acta Orthop 59:151–154
Tatar Z, Soubrier M, Dillies AF et al (2014) Assessment of the risk factors for impending fractures following radiotherapy for long bone metastases using CT scan-based virtual simulation: a retrospective study. Radiat Oncol 9:227
Groenen KH, Pouw MH, Hannink G et al (2016) The effect of radiotherapy, and radiotherapy combined with bisphosphonates or RANK ligand inhibitors on bone quality in bone metastases. A systematic review. Radiother Oncol 119:194–201
Acknowledgment
We would like to thank Editage (www.editage.jp) for English language editing.
Funding
This work was supported by KAKENHI (Grants-in-Aid for Scientific Research (C)) Grant Number 15K10388.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: YS, HK; Collection and assembly of data: YS, RS, YI, TA, LZ, TH, MI, KO, TO; Analysis and interpretation of data: YS, HO, HK; Drafting of the article: YS; Critical revision of the article for important intellectual content: TA, HK, TG, ST; Final approval of the article: NH.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest regarding the publication of this paper.
Ethical approval
This study was conducted according to the ethical guidelines for epidemiological research laid out by the Japanese Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labour and Welfare. The study protocol was included in a comprehensive protocol developed by the Department of Rehabilitation and the Department of Orthopaedic Surgery, the University of Tokyo Hospital. The study was approved by the University of Tokyo Medical Research Center Ethics Committee (approval number: 2373).
Consent to participate
All participants were provided a means to opt out.
Availability of data and material
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Shinoda, Y., Sawada, R., Ishibashi, Y. et al. Prediction of pathological fracture in patients with lower limb bone metastasis using computed tomography imaging. Clin Exp Metastasis 37, 607–616 (2020). https://doi.org/10.1007/s10585-020-10053-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-020-10053-z