Abstract
Locoregionally advanced and unresectable disease can be seen in up to 10% of melanoma patients. Treatment options for these patients have been evolving most notably over the past few decades and have demonstrated efficacy through multiple intra-arterial as well as intralesional therapies. Isolated limb perfusions and isolated limb infusions have been utilized to treat locoregionally advanced melanoma of the extremity with overall response rates up to 90% in some reports. Intralesional therapies, for in transit metastatic melanoma, such as Bacille Calmette–Guerin, talimogene laherparepvec, and PV-10 (Rose Bengal) have all demonstrated efficacy in the treatment of unresectable cutaneous melanoma. The treatment effect due to intralesional injection has been identified in directly injected lesions as well as in distant uninjected “bystander lesions” with some injectables. This bystander effect is likely an immunologic reaction due to tumor antigen release, antigen-presenting cell uptake, T cell activation and subsequent bystander tumor destruction in uninjected lesions. Treatment options for unresectable melanoma metastases limited to the liver include isolated hepatic perfusion, which can now be performed through a minimally invasive approach known as percutaneous hepatic perfusion. These intra-arterial and intralesional regional therapies offer a variety of effective treatment modalities for unresectable disease and may potentially be combined with systemic treatments, such as immunotherapy, in the future treatment of locoregionally advanced melanoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of cutaneous melanoma is on the rise by approximately 3–8% annually over the last several decades [1, 2]. The American Cancer Society estimates upwards of 87,000 new melanoma cases in the United States and roughly 10,000 deaths due to melanoma in 2017 [2]. On initial clinical presentation, approximately 84% of melanoma patients present with localized disease, 9% present with regional disease (including satellite lesions, in-transit disease and regional nodal involvement), and 4% present with distant metastatic disease [2]. 5-year melanoma-specific survival (MSS) rates are significantly impacted by stage ranging from 98% for stage I, 90% for stage II and 77% for stage III disease, respectively. 5-year overall survival (OS) for stage IV patients still is dismal at approximately 10% [3].
Early stage melanoma is typically treated by wide excision and often accompanied by sentinel lymph node biopsy [4]; however, patients with advanced locoregional disease are often unresectable due to extensive satellitosis or in-transit lesions. The treatment of unresectable regional disease has been evolving and improving over the past century through advancements in intra-arterial and intralesional therapies. Intra-arterial therapies have been developed to target disease most commonly limited to the extremities as well as the liver; whereas intralesional therapies have been utilized primarily for the management of locoregionally advanced cutaneous lesions.
Intra-arterial therapies
In 1958 Creech et al. developed isolated limb perfusion (HILP), a technique that allows for perfusion of an isolated extremity with high-dose chemotherapy [5]. HILP utilizes a cardiopulmonary bypass machine to infuse and circulate chemotherapy via directly cannulated major vessels of an isolated limb. In 1967, Cavaliere et al. introduced a heat exchanger to the extracorporeal circuit for hyperthermic isolated limb perfusion (HILP), which was further refined by Stehlin in 1975 [6, 7]. During HILP, melphalan-based chemotherapy is typically circulated for 60 min at flow rates of 400 to 600 mL/min to the isolated limb at temperatures of 39–41 °C in an oxygenated environment while tying off of collaterals and the use of a tourniquet is used to prevent backflow of chemotherapy into the systemic circulation [8, 9].
HILP has been successfully applied to the treatment of locoregionally advanced melanoma, specifically targeting in-transit disease of an extremity. The overall response rate (ORR) to HILP has been reported as high as 80–90% and complete responses (CR) have been reported around 40–70% [9,10,11,12,13]. However, HILP is associated with significant morbidity, most notably with lymphedema seen in up to 36% of patients [14]. Additionally, due to open surgical cannulation and the high rates of subsequent lymphedema, HILP is typically only performed once on a given patient. Given the morbidity associated with the open technique of HILP, there was interest in adapting the technique to a minimally invasive approach.
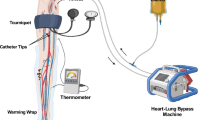
Isolated limb infusion (ILI), a minimally invasive modification of HILP, was first adapted by Thompson et al. in the 1990s to treat in-transit, unresectable melanoma isolated to one limb [15]. ILI utilizes arterial and venous catheters to isolate the inflow and outflow of the limb. The limb is heated to at least 37 °C and the patient is heparinized to achieve an activated clotting time (ACT) ≥ 350. ILI utilizes lower flow rates than HILP (typically 80–120 mL/min) and is performed under hyperthermic temperatures in an anaerobic environment (Fig. 1). Similar to HILP, ILI utilizes Melphalan-based chemotherapy dosed at 7.5 mg/L for the lower extremity and 10 mg/L for the upper extremity with maximum doses of 100 and 50 mg for the lower and upper extremities, respectively. Melphalan doses are calculated using ideal body weight (IBW) with the followed formula: Melphalan dose per liter of limb volume to be infused (mg/L) × calculated volume of extremity (L) × ideal body weight (kg) ÷ actual body weight (kg) [16, 17].
ILI has since been expanded to include the treatment of sarcoma and other cutaneous malignancies [18]. Regional complications according to the Wieberdink (WBD) scale have been reported with ILI, and are usually mild to moderate including localized erythema and edema of the skin [19]. However, ILI typically tends to be less morbid and better tolerated than HILP due to its minimally invasive approach. Single and multi-institutional data for melanoma patients treated with ILI demonstrated an ORR around 60% with a CR around 30% [20,21,22].
In a single institutional series from Moffitt Cancer Center, a total of 205 ILI procedures were attempted, with 201 successfully performed on 164 patients [21]. 145 of those procedures were performed for melanoma. 114 of those were performed as an initial ILI (82% lower extremity, 18% upper extremity) and 31 were performed as repeat ILI’s (79% lower extremity, 21% upper extremity). The median WBD score was 2 (range 1–4). Post-ILI creatine phosphokinase (CPK) values peaked at 386 and 1381 IU/L for upper extremity and lower extremity procedures, respectively. At 3 months, ORR was 59.2%, (including a 26.1% CR), 12.7% of patients demonstrated stable disease and 28.2% of patients had progression of disease. When stratified by extremity type, ILI for upper extremity demonstrated a 76.9% ORR and lower extremity demonstrated a 55.1% ORR (p = 0.04). Initial ILI demonstrated an ORR of 58.4% and repeat ILI demonstrated an ORR of 60.0% (p = 0.7). 26% of patients that achieved a partial response (PR) with ILI subsequently underwent completion resection to have no evidence of disease (NED). Response to ILI demonstrated benefits for both in-field progression-free survival (IPFS; 14.1 vs 3.2 months, p < 0.0001) as well as OS (56.0 vs 26.7 months, p = 0.0004) when compared to non-responders. Median time to distant metastatic-free survival was not reached for responders as compared to 25.5 months for non-responders (p = 0.018).
Minimally invasive approaches, as seen in the treatment of locoregional limb disease, have also been utilized in the setting of isolated hepatic melanoma metastases. There are myriad treatment options for liver metastases from melanoma including surgical resection, isolated hepatic perfusion (IHP), catheter-directed therapies [transarterial chemoembolization (TACE), radiofrequency ablation (RFA), Yttrium beads], cryotherapy, radiation therapy and systemic therapy. More recently, percutaneous hepatic perfusion (PHP) has been utilized for unresectable metastatic melanoma to the liver. PHP utilizes a minimally invasive percutaneous approach to achieve hepatic vascular isolation and deliver high-dose chemotherapy to liver metastases. Melphalan is typically perfused at 3 mg/kg, corrected for IBW, and delivered via selective cannulation of the left and right hepatic arteries. Hepatic vascular occlusion and veno–veno bypass is used to minimize systemic perfusion. The technique is commonly used for metastatic ocular melanoma, and the PHPs are typically repeated every 6–8 weeks for up to 6 total treatments if there is a response, limited adverse effects (recovery from any adverse effects between treatments) and no extrahepatic disease development [23].
Only approximately 20% of primary cutaneous melanoma metastases involve the liver; however, metastases from uveal melanoma involve the liver in 50–80% of cases and in most of those cases the liver is the only involved site [24]. Metastatic uveal melanoma carries a particularly dismal prognosis with 1-year survival rates reported at 10–25% [25,26,27,28]. Median survival has been reported up to 27 months in cases of resectable ocular melanoma liver metastases [29], In comparison, unresectable disease is typically refractory to systemic therapy and median survival has been reported at 4–5 months [30,31,32].
Forster et al. retrospectively reviewed a series of patients with unresectable melanoma and sarcoma liver metastases to evaluate therapeutic response, morbidity, hepatic progression-free survival (hPFS) and OS in the setting of PHP [27]. A total of 27 PHPs were performed on ten patients. Five of the patients had metastatic ocular melanoma, three had cutaneous melanoma, one had an unknown primary melanoma and one had a sarcoma. 90% of patients demonstrated stable disease or PR to treatment and the median hPFS was 240 days. There were no perioperative mortalities and myelosuppression was the most common morbidity associated with PHP.
Hughes et al. reported their results of a randomized, controlled, multicenter phase III trial comparing PHP with best available care (BAC) for patients with melanoma liver metastases [23]. 93 patients with metastatic melanoma were randomized to either PHP or BAC. The primary endpoint was hPFS and secondary endpoints were overall progression-free survival (oPFS), OS, hepatic objective response (hOR), and safety. 49 patients received 115 PHP procedures (median number of PHPs per patient = 2). 85% of treated patients had intrahepatic disease only. Roughly 1/3 of patients had either previous liver-directed treatment or previous systemic treatment. After a median follow-up of 294 days, median hPFS in the PHP group was 7.0 months (95% CI 5.2–9.7 months) vs 1.6 months (95% CI 1.5–2.9 months) in the BAC group (p < 0.0001). Median oPFS in the PHP group was 5.4 months (95% CI 3.4–8.1 months) vs 1.6 months (95% CI 1.5–2.3 months) in the BAC group (p = 0.0001). OS was not significantly improved in the PHP arm (10.6 months for PHP vs 10.0 months for BAC); however, this was likely attributable to the high crossover rate of 57.1% from the BAC arm to PHP after progression of disease on BAC. 1-year hPFS for PHP responders was 45.9 and 18.9% for those patients with stable disease after PHP. Similar benefits were appreciated on OS stratified by hepatic response with a 2-year OS of 60% for responders vs 21% for non-responders (p = 0.0061). OS was also impacted by disease burden with a more favorable prognosis for those patients who had a low burden of disease as defined by ≤ 10 lesions and < 50% liver involvement (p = 0.02). The most common adverse events were hematologic and cardiovascular, likely related to melphalan perfusion and bypass for liver isolation, respectively.
Intralesional therapies
Intra-arterial therapies have been effectively complemented by numerous intralesional therapies that have been utilized in the treatment of locoregionally advanced melanoma. The concept of intralesional therapies for cancer treatment was first reported in 1893 by Coley who injected malignant tumors with repeated inoculations of erysipelas [33]. Many of the current intralesional therapies demonstrate efficacy, in part, by mediating a T cell response in the host tumor bed with the potential for clinical impact on more distant sites of disease due to the immune response. Historically, Bacille Calmette–Guerin (BCG) is the most recognized intralesional therapy used for in-transit melanoma and was first reported by Dr. Morton et al. in 1974 [34]. That initial series demonstrated 90% regression of the injected cutaneous lesions as well as 17% regression of uninjected lesions. Disease-free survival was sustained up to 74 months after injection in select patients. However, later studies by the Eastern Cooperative Oncology Group (ECOG) did not demonstrate any survival differences between patients that received either BCG, BCG with dacarbazine, or observation alone. Additionally, the use of BCG was associated with the development of punctate abscesses in greater than two-thirds of the patients treated in this study [35].
Talimogene laherparepvec (T-VEC) is an oncolytic herpes simplex type 1 virus (HSV-1) that has been modified to selectively replicate within tumors and produce granulocyte macrophage colony-stimulating factor (GM-CSF). Genetic deletion of two non-essential HSV-1 genes reduces the suppression of antigen presentation and enables the virus to proliferate in tumor cells. Further viral modification by insertion of the gene for GM-CSF promotes an immunologic response in the tumor microenvironment through increased dendritic cell activity, heightened T cell responses and further increased tumor antigen presentation [36,37,38]. T-VEC is approved for intralesional injection of cutaneous, subcutaneous and nodal melanoma. Phase I, II, and III clinical trials have been conducted to evaluate the safety and efficacy of T-VEC. Treatment protocols are typically characterized by intralesional injections with up to 4 mL of 106 PFU/mL followed by up to 4 mL of 108 PFU/mL every 2 weeks for up to 24 total treatments [38, 39]. Typical side-effects include local reactions at the injection site as well as flu-like symptoms.
Initial phase II clinical trials demonstrated an ORR of 26% (including a 16% CR) with some durable responses that lasted up to 31 months as well as some improvement in uninjected visceral bystander lesions (Fig. 2). 1- and 2-year OS were reported at 58 and 52%, respectively [40]. In a phase III clinical trial, investigators compared intralesional T-VEC to subcutaneously injected GM-CSF for unresectable stage IIIB to IV melanoma patients to assess durable response rate (DRR; defined as a partial or CR lasting continuously ≥ 6 months), OS, and ORR [41]. 436 patients were randomly assigned at a 2:1 ratio (T-VEC: GM-CSF). T-VEC demonstrated a significant advantage compared to GM-CSF with a DRR of 16.3% compared to 2.1%, respectively. ORR was also significantly improved in the T-VEC arm (26.4%) compared to the GM-CSF arm (5.7%). Median OS was trending towards demonstrating benefit in the T-VEC arm at 23.3 vs 18.9 months (p = 0.051). On subgroup analysis, TVEC demonstrated an improvement in DRR for patients with stage IIIB or IIIC disease (33 vs 0%) as well as patients with stage IVM1a disease (16 vs 2%). TVEC also demonstrated an improvement in ORR for patients with stage IIIB or IIIC disease (52.3 vs 2.3%) as well as patients with stage IVM1a disease (26.7 vs 2.3%).
The investigators additionally reported on the clinical pattern of response to T-VEC by assessing lesion-level gross response as well as the pathophysiologic impact of T-VEC treatment utilizing immunohistochemistry (IHC) [42]. T-VEC treatment resulted in at least 50% reduction in the size of 64% of injected lesions, 34% of uninjected non-visceral and 15% of visceral bystander lesions. Complete resolution was noted in 47% of injected lesions, 22% of uninjected non-visceral and 9% of visceral bystander lesions. On histologic assessment T-VEC demonstrated a dramatic destruction of malignant melanoma cell populations as well as an influx of lymphocyte populations in response to local TVEC injection (Fig. 3). These results have also been demonstrated in the setting of melanoma that has been refractory to prior treatment including ILI, chemotherapy and systemic immunotherapy. Due to these findings, TVEC was approved by the FDA for use for stage IIIB, IIIC, and IVM1a melanoma in October 2015.
PV-10, also known as rose bengal, is another intralesional therapy that has demonstrated promising results in the treatment of locoregionally advanced cutaneous melanoma. PV-10 is a 10% solution of rose bengal, which is a water-soluble xanthine dye that was initially used to diagnose ophthalmologic damage and liver cancer [43]. The mechanism of action is attributed to the xanthine dye, which facilitates the formation of reactive oxygen species with visible light and thereby initiates a phototoxic effect. The lysosomes of cancer cells selectively absorb the xanthine dye allowing for a relatively targeted treatment effect. PV-10 targets tumor cells via two mechanisms: (1) primary ablation due to intralesional injection and (2) a secondary immunomodulatory effect is seen upon tumor antigen release, antigen-presenting cell (APC) uptake, T cell activation and subsequent bystander tumor destruction in uninjected lesions [44, 45]. Due to these mechanisms, PV-10 has demonstrated efficacy in the treatment of satellite and in-transit disease in both injected and bystander lesions.
A phase I trial on PV-10 demonstrated an ORR of 48% for injected lesions as well as a 27% ORR for injected bystander lesions; however, this study only assessed 11 patients [43]. There was a dose-dependent effect of PV-10 seen in this study with lower response rates seen in lesions that received less than 0.2 mL/cc per lesion compared to 0.5 mL/cc per lesion. Another phase I study utilized a single dose of PV-10 and again demonstrated comparable results with an OR of 40% including a 20% response in injected lesions and 15% response in bystander lesions [46]. A follow-up phase II trial was conducted on 80 patients that demonstrated equally promising results [46]. In this study, up to 20 lesions were injected with repeat injections at 8, 12, and 16 weeks if needed. Most patients responded after the initial injection. An ORR of 51% was seen, including a 26% CR. Uninjected lesions demonstrated a 33% ORR (26% CR, 7% PR). Adverse effects were most commonly related to injection site pain, erythema, swelling, and photosensitivity.
Intra-arterial and intralesional therapies have been effectively utilized in the treatment of locoregionally advanced melanoma; however, the optimal use of locoregional therapies in the setting of targeted therapy and systemic checkpoint blockade has not been well established. Intralesional treatments, such as T-VEC, are oncolytic therapies that typically induce an elevated immune response [34, 37]. Therefore, the combination of locoregional therapies with targeted or systemic immunotherapies could potentially demonstrate a synergistic effect [47]. There are currently multiple clinical trials that are ongoing and others that have shown early promise in a multi-modality approach for locoregionally advanced and metastatic disease [48].
Ipilimumab in combination with T-VEC has been evaluated in a phase II clinical trial and has demonstrated an ORR of 50% (compared to up to 11.9% for ipilimumab alone [49] and 26% for T-VEC alone [42]) and a 22% CR with durability beyond 1 year [50]. T-VEC in combination with pembrolizumab is currently being evaluated in a phase I/III clinical trial and early data from the trial has demonstrated an ORR of 57% (compared to approximately 34% for pembrolizumab alone [49] and 26% for T-VEC alone [42]) with a CR of 24% [51]. A multi-center, international phase Ib/II study is currently underway to assess the safety and efficacy of PV-10 plus pembrolizumab for the treatment of metastatic melanoma (NCT02557321). Additionally, a multi-center, international phase III study is currently comparing PV-10 vs chemotherapy vs T-VEC to assess progression-free survival, CR rate, duration of CR and OS in patients with stage IIIB, IIIC and IV M1a melanoma (NCT02288897). Further studies will elucidate the role of combination therapy, including a staged approach using upfront locoregional therapies to prime the immune system prior to systemic immunotherapy. The rapidly changing landscape of immunotherapy-based melanoma treatment will likely be enhanced by this multimodality approach.
Conclusion
Melanoma presents as locoregionally advanced unresectable disease in up to 10% of patients and is a particularly challenging spectrum of disease to manage; however, there are still numerous options that can be used as monotherapy or potentially in combination. These options include intra-arterial therapies, such as ILI and PHP, and intralesional treatments, such as BCG, TVEC and PV-10. These locoregional therapies are typically well tolerated, easily delivered and have shown significant promise in the treatment of locoregionally advanced melanoma.
Abbreviations
- HILP:
-
Isolated limb perfusions
- ILI:
-
Isolated limb infusions
- BCG:
-
Bacille Calmette–Guerin
- T-VEC:
-
Talimogene laherparepvec
- APC:
-
Antigen-presenting cell
- PHP:
-
Percutaneous hepatic perfusion
- MSS:
-
Melanoma-specific survival
- OS:
-
Overall survival
- ORR:
-
Overall response rate
- CR:
-
Complete responses
- ACT:
-
Activated clotting time
- IBW:
-
Ideal body weight
- WBD:
-
Wieberdink
- CPK:
-
Creatine phosphokinase
- PR:
-
Partial response
- NED:
-
No evidence of disease
- IPFS:
-
In-field progression-free survival
- IHP:
-
Isolated hepatic perfusion
- TACE:
-
Transarterial chemoembolization
- RFA:
-
Radiofrequency ablation
- hPFS:
-
Hepatic progression-free survival
- BAC:
-
Best available care
- oPFS:
-
Overall progression-free survival
- hOR:
-
Hepatic objective response
- ECOG:
-
Eastern Cooperative Oncology Group
- HSV-1:
-
Herpes simplex type 1 virus
- GM-CSF:
-
Granulocyte macrophage colony-stimulating factor
- DRR:
-
Durable response rate
- IHC:
-
Immunohistochemistry
References
Thompson JF, Scolyer RA, Kefford RF (2005) Cutaneous melanoma. Lancet 365(9460):687–701. https://doi.org/10.1016/S0140-6736(05)17951-3
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67(1):7–30. https://doi.org/10.3322/caac.21387
Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, Lazar AJ, Faries MB, Kirkwood JM, McArthur GA, Haydu LE, Eggermont AMM, Flaherty KT, Balch CM, Thompson JF, for members of the American Joint Committee on Cancer Melanoma Expert Panel, the International Melanoma Database, Discovery Platform (2017) Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 67(6):472–492. https://doi.org/10.3322/caac.21409
Han D, Yu D, Zhao X, Marzban SS, Messina JL, Gonzalez RJ, Cruse CW, Sarnaik AA, Puleo C, Sondak VK, Zager JS (2012) Sentinel node biopsy is indicated for thin melanomas ≥ 0.76 mm. Ann Surg Oncol 19(11):3335–3342. https://doi.org/10.1245/s10434-012-2469-1
Creech O Jr, Krementz ET, Ryan RF, Winblad JN (1958) Chemotherapy of cancer: regional perfusion utilizing an extracorporeal circuit. Ann Surg 148(4):616–632
Cavaliere R, Ciocatto EC, Giovanella BC, Heidelberger C, Johnson RO, Margottini M, Mondovi B, Moricca G, Rossi-Fanelli A (1967) Selective heat sensitivity of cancer cells. Biochemical and clinical studies. Cancer 20(9):1351–1381
Stehlin JS, Giovanella BC, de Ipolyi PD, Muenz LR, Anderson RF (1975) Results of hyperthermic perfusion for melanoma of the extremities. Surg Gynecol Obstet 140(3):339–348
Fraker DL (1999) Hyperthermic regional perfusion for melanoma and sarcoma of the limbs. Curr Probl Surg 36(11):841–907
Fraker DL (2004) Management of in-transit melanoma of the extremity with isolated limb perfusion. Curr Treat Options Oncol 5(3):173–184
Noorda EM, Vrouenraets BC, Nieweg OE, van Geel BN, Eggermont AM, Kroon BB (2004) Isolated limb perfusion for unresectable melanoma of the extremities. Arch Surg 139(11):1237–1242. https://doi.org/10.1001/archsurg.139.11.1237
Kroon BB, Noorda EM, Vrouenraets BC, Nieweg OE (2002) Isolated limb perfusion for melanoma. J Surg Oncol 79(4):252–255. https://doi.org/10.1002/jso.10077
Eggermont AM, Schraffordt Koops H, Klausner JM, Kroon BB, Schlag PM, Lienard D, van Geel AN, Hoekstra HJ, Meller I, Nieweg OE, Kettelhack C, Ben-Ari G, Pector JC, Lejeune FJ (1996) Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas. The cumulative multicenter European experience. Ann Surg 224(6):756–764 (discussion 764–755)
Cornett WR, McCall LM, Petersen RP, Ross MI, Briele HA, Noyes RD, Sussman JJ, Kraybill WG, Kane JM 3rd, Alexander HR, Lee JE, Mansfield PF, Pingpank JF, Winchester DJ, White RL Jr, Chadaram V, Herndon JE 2nd, Fraker DL, Tyler DS, American College of Surgeons Oncology Group Trial Z (2006) Randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone compared with melphalan plus tumor necrosis factor: American College of Surgeons Oncology Group Trial Z0020. J Clin Oncol 24(25):4196–4201. https://doi.org/10.1200/JCO.2005.05.5152
Moller MG, Lewis JM, Dessureault S, Zager JS (2008) Toxicities associated with hyperthermic isolated limb perfusion and isolated limb infusion in the treatment of melanoma and sarcoma. Int J Hyperth 24(3):275–289. https://doi.org/10.1080/02656730701805520
Thompson JF, Kam PC, Waugh RC, Harman CR (1998) Isolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusion. Semin Surg Oncol 14(3):238–247
Beasley GM, Petersen RP, Yoo J, McMahon N, Aloia T, Petros W, Sanders G, Cheng TY, Pruitt SK, Seigler H, Tyler DS (2008) Isolated limb infusion for in-transit malignant melanoma of the extremity: a well-tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol 15(8):2195–2205. https://doi.org/10.1245/s10434-008-9988-9
Santillan AA, Zager JS (2010) Isolated limb infusion for melanoma: a less morbid alternative to hyperthermic isolated limb perfusion in the US. Expert Opin Drug Metab Toxicol 6(9):1033–1037. https://doi.org/10.1517/17425250903559881
Wong J, Chen YA, Fisher KJ, Zager JS (2013) Isolated limb infusion in a series of over 100 infusions: a single-center experience. Ann Surg Oncol 20(4):1121–1127. https://doi.org/10.1245/s10434-012-2782-8
Wieberdink J, Benckhuysen C, Braat RP, van Slooten EA, Olthuis GA (1982) Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol 18(10):905–910
Beasley GM, Caudle A, Petersen RP, McMahon NS, Padussis J, Mosca PJ, Zager JS, Hochwald SN, Grobmyer SR, Delman KA, Andtbacka RH, Noyes RD, Kane JM, Seigler H, Pruitt SK, Ross MI, Tyler DS (2009) A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg 208(5):706–715. https://doi.org/10.1016/j.jamcollsurg.2008.12.019 (discussion 715–707)
O’Donoghue C, Perez MC, Mullinax JE, Hardman D, Sileno S, Naqvi SMH, Kim Y, Gonzalez RJ, Zager JS (2017) Isolated limb infusion: a single-center experience with over 200 infusions. Ann Surg Oncol 24(13):3842–3849. https://doi.org/10.1245/s10434-017-6107-9
Kroon HM, Coventry BJ, Giles MH, Henderson MA, Speakman D, Wall M, Barbour A, Serpell J, Paddle P, Smithers BM, Thompson JF (2017) Safety and efficacy of isolated limb infusion chemotherapy for advanced locoregional melanoma in elderly patients: an Australian multicenter study. Ann Surg Oncol 24(11):3245–3251. https://doi.org/10.1245/s10434-017-6046-5
Hughes MS, Zager J, Faries M, Alexander HR, Royal RE, Wood B, Choi J, McCluskey K, Whitman E, Agarwala S, Siskin G, Nutting C, Toomey MA, Webb C, Beresnev T, Pingpank JF (2016) Results of a randomized controlled multicenter phase III trial of percutaneous hepatic perfusion compared with best available care for patients with melanoma liver metastases. Ann Surg Oncol 23(4):1309–1319. https://doi.org/10.1245/s10434-015-4968-3
Abbott AM, Doepker MP, Kim Y, Perez MC, Gandle C, Thomas KL, Choi J, Shridhar R, Zager JS (2017) Hepatic progression-free and overall survival after regional therapy to the liver for metastatic melanoma. Am J Clin Oncol. https://doi.org/10.1097/COC.0000000000000356
Feldman ED, Pingpank JF, Alexander HR Jr (2004) Regional treatment options for patients with ocular melanoma metastatic to the liver. Ann Surg Oncol 11(3):290–297
Pereira PR, Odashiro AN, Lim LA, Miyamoto C, Blanco PL, Odashiro M, Maloney S, De Souza DF, Burnier MN Jr (2013) Current and emerging treatment options for uveal melanoma. Clin Ophthalmol 7:1669–1682. https://doi.org/10.2147/OPTH.S28863
Forster MR, Rashid OM, Perez MC, Choi J, Chaudhry T, Zager JS (2014) Chemosaturation with percutaneous hepatic perfusion for unresectable metastatic melanoma or sarcoma to the liver: a single institution experience. J Surg Oncol 109(5):434–439. https://doi.org/10.1002/jso.23501
Rashid OM, Sloot S, Zager JS (2014) Regional therapy in metastatic melanoma: an update on minimally invasive intraarterial isolated limb infusion and percutaneous hepatic perfusion. Expert Opin Drug Metab Toxicol 10(10):1355–1364. https://doi.org/10.1517/17425255.2014.951330
Mariani P, Piperno-Neumann S, Servois V, Berry MG, Dorval T, Plancher C, Couturier J, Levy-Gabriel C, Lumbroso-Le Rouic L, Desjardins L, Salmon RJ (2009) Surgical management of liver metastases from uveal melanoma: 16 years’ experience at the Institut Curie. Eur J Surg Oncol 35(11):1192–1197. https://doi.org/10.1016/j.ejso.2009.02.016
Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, Hawkins BS, Hayman JA, Jaiyesimi I, Jampol LM, Kirkwood JM, Koh WJ, Robertson DM, Shaw JM, Straatsma BR, Thoma J, Collaborative Ocular Melanoma Study Group (2005) Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group report no. 26. Arch Ophthalmol 123(12):1639–1643. https://doi.org/10.1001/archopht.123.12.1639
Gragoudas ES, Egan KM, Seddon JM, Glynn RJ, Walsh SM, Finn SM, Munzenrider JE, Spar MD (1991) Survival of patients with metastases from uveal melanoma. Ophthalmology 98(3):383–389 (discussion 390)
Singh AD, Borden EC (2005) Metastatic uveal melanoma. Ophthalmol Clin North Am 18(1):143–150. https://doi.org/10.1016/j.ohc.2004.07.003
Coley WB (1991) The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res (262):3–11
Morton DL, Eilber FR, Holmes EC, Hunt JS, Ketcham AS, Silverstein MJ, Sparks FC (1974) BCG immunotherapy of malignant melanoma: summary of a seven-year experience. Ann Surg 180(4):635–643
Agarwala SS, Neuberg D, Park Y, Kirkwood JM (2004) Mature results of a phase III randomized trial of bacillus Calmette–Guerin (BCG) versus observation and BCG plus dacarbazine versus BCG in the adjuvant therapy of American Joint Committee on Cancer Stage I–III melanoma (E1673): a trial of the Eastern Oncology Group. Cancer 100(8):1692–1698. https://doi.org/10.1002/cncr.20166
He B, Chou J, Brandimarti R, Mohr I, Gluzman Y, Roizman B (1997) Suppression of the phenotype of gamma(1)34.5-herpes simplex virus 1: failure of activated RNA-dependent protein kinase to shut off protein synthesis is associated with a deletion in the domain of the alpha47 gene. J Virol 71(8):6049–6054
Liu BL, Robinson M, Han ZQ, Branston RH, English C, Reay P, McGrath Y, Thomas SK, Thornton M, Bullock P, Love CA, Coffin RS (2003) ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther 10(4):292–303. https://doi.org/10.1038/sj.gt.3301885
Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G, Gonzalez R, Glaspy J, Whitman E, Harrington K, Goldsweig H, Marshall T, Love C, Coffin R, Nemunaitis JJ (2009) Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol 27(34):5763–5771. https://doi.org/10.1200/JCO.2009.24.3675
Hu JC, Coffin RS, Davis CJ, Graham NJ, Groves N, Guest PJ, Harrington KJ, James ND, Love CA, McNeish I, Medley LC, Michael A, Nutting CM, Pandha HS, Shorrock CA, Simpson J, Steiner J, Steven NM, Wright D, Coombes RC (2006) A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res 12(22):6737–6747. https://doi.org/10.1158/1078-0432.CCR-06-0759
Johnson DB, Puzanov I, Kelley MC (2015) Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma. Immunotherapy 7(6):611–619. https://doi.org/10.2217/imt.15.35
Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, Milhem M, Cranmer L, Curti B, Lewis K, Ross M, Guthrie T, Linette GP, Daniels GA, Harrington K, Middleton MR, Miller WH Jr, Zager JS, Ye Y, Yao B, Li A, Doleman S, VanderWalde A, Gansert J, Coffin RS (2015) Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 33(25):2780–2788. https://doi.org/10.1200/JCO.2014.58.3377
Andtbacka RH, Ross M, Puzanov I, Milhem M, Collichio F, Delman KA, Amatruda T, Zager JS, Cranmer L, Hsueh E, Chen L, Shilkrut M, Kaufman HL (2016) Patterns of clinical response with talimogene laherparepvec (T-VEC) in patients with melanoma treated in the OPTiM phase III clinical trial. Ann Surg Oncol 23(13):4169–4177. https://doi.org/10.1245/s10434-016-5286-0
Thompson JF, Hersey P, Wachter E (2008) Chemoablation of metastatic melanoma using intralesional Rose Bengal. Melanoma Res 18(6):405–411. https://doi.org/10.1097/CMR.0b013e32831328c7
Foote MC, Burmeister BH, Thomas J, Mark Smithers B (2010) A novel treatment for metastatic melanoma with intralesional rose bengal and radiotherapy: a case series. Melanoma Res 20(1):48–51. https://doi.org/10.1097/CMR.0b013e328331caa2
Toomey P, Kodumudi K, Weber A, Kuhn L, Moore E, Sarnaik AA, Pilon-Thomas S (2013) Intralesional injection of rose bengal induces a systemic tumor-specific immune response in murine models of melanoma and breast cancer. PLoS ONE 8(7):e68561. https://doi.org/10.1371/journal.pone.0068561
Thompson JF, Agarwala SS, Smithers BM, Ross MI, Scoggins CR, Coventry BJ, Neuhaus SJ, Minor DR, Singer JM, Wachter EA (2015) Phase 2 study of intralesional PV-10 in refractory metastatic melanoma. Ann Surg Oncol 22(7):2135–2142. https://doi.org/10.1245/s10434-014-4169-5
Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J, Fernandez E, Kirkwood JM, Gajewski TF, Chen L, Gorski KS, Anderson AA, Diede SJ, Lassman ME, Gansert J, Hodi FS, Long GV (2017) Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 170(6):1109–1119. https://doi.org/10.1016/j.cell.2017.08.027
Dummer R, Hoeller C, Gruter IP, Michielin O (2017) Combining talimogene laherparepvec with immunotherapies in melanoma and other solid tumors. Cancer Immunol Immunother 66(6):683–695. https://doi.org/10.1007/s00262-017-1967-1
Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank C, Petrella TM, Hamid O, Zhou H, Ebbinghaus S, Ibrahim N, Robert C (2017) Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 390(10105):1853–1862. https://doi.org/10.1016/S0140-6736(17)31601-X
Puzanov I, Milhem MM, Minor D, Hamid O, Li A, Chen L, Chastain M, Gorski KS, Anderson A, Chou J, Kaufman HL, Andtbacka RH (2016) Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J Clin Oncol 34(22):2619–2626. https://doi.org/10.1200/JCO.2016.67.1529
Long GV (2016) Efficacy analysis of MASTERKEY-265 phase 1b study of talimogene laherparepvec (T-VEC) and pembrolizumab (pe,bro) for unresectable stage IIIB-IV melanoma. J Clin Oncol 34(suppl):Abstract 9568
Funding
This study was supported by "Provectus Biopharmaceuticals, Delcath Systems".
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weitman, E.S., Zager, J.S. Regional therapies for locoregionally advanced and unresectable melanoma. Clin Exp Metastasis 35, 495–502 (2018). https://doi.org/10.1007/s10585-018-9890-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-018-9890-1