Abstract
Survival among patients with metastatic breast cancer may vary according to the site of metastasis and receptor status. We used Danish nationwide medical registries to establish a cohort of patients with metastatic breast cancer (870 with de novo metastatic disease and 3518 with recurrent disease with distant metastasis) diagnosed during 1997–2011. We examined 1-year and >1 to 5-year mortality associated with first site of metastasis and receptor expression status of the primary tumor. Cox proportional regression was used to compute confounder-adjusted mortality rate ratios (MRRs) associated with site of metastasis, stratified by receptor status. Overall 1-year and >1 to 5-year mortality risks were 36 and 69 %, respectively. Risk of death within 1 year was highest for brain-only (62 %) and liver-only (43 %) involvement and nearly the same for patients with lung-only (32 %), bone-only (32 %) involvement, and other/combination of sites (34 %). Using bone-only metastasis as reference, women with brain-only metastasis had more than two-fold increased risk of dying. The adjusted MRR for women with liver-only metastasis also was increased, though less pronounced. Patients with lung-only [adjusted MRR 0.9 (95 % confidence interval (CI) 0.8, 1.1)] or other metastases [adjusted MRR 1.0 (95 % CI 0.9, 1.2)] had similar mortality as patients with bone-only metastasis. Positive hormonal receptor status was a favorable prognostic factor. Metastatic breast cancer has a serious prognosis. Patients with brain-only metastasis had the highest mortality. Positive hormonal receptor status on the primary tumor was a favorable prognostic factor for all metastatic sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Up to 10 % of women with breast cancer have distant metastasis at diagnosis (de novo metastatic disease) [1], and nearly half of patients with early-stage disease treated with post-operative adjuvant therapy experience a recurrence, peaking 2 years after diagnosis [2]. Older data suggest that median survival after breast cancer metastasis ranges between 18 and 24 months [3, 4]. Recently, survival has improved for de novo metastatic disease, mainly among younger women, with more widespread use of novel oncology drugs such as monoclonal antibodies [5–7]. Median survival may be similar or nearly 12 months shorter among patients with recurrent distant breast cancer than in patients with de novo metastatic disease [5, 8, 9]. A longer disease-free interval from primary diagnosis to metastasis may be associated with more favorable overall survival after recurrence [6, 8, 9].
Specific biological breast cancer subtypes are characterized by different patterns of metastatic spread to various organs, with differential impacts on survival [10]. Hormonal receptor-positive tumors often metastasize to the bone, [10] and patients with such metastases may survive longer than patients with brain or visceral metastases [5, 8–11]. Thus expression of hormone-positive receptors on the primary tumor are associated with better overall survival after metastasis [5, 6, 10].
Few data are available on the impact of metastatic site on mortality. We therefore conducted a nationwide population-based cohort study of patients with metastatic breast cancer to examine the associations between site of metastasis, receptor expression, and mortality.
Methods
Source population and data collection
We designed this population-based cohort study to examine mortality following a diagnosis of metastatic breast cancer, based on nationwide Danish medical and administrative registries. The Danish tax-funded national health care system provides access free of charge for the entire population of approximately 5.6 million people [12]. Within this system, medical and administrative registries can be used to track diagnoses, procedures, treatments, and vital status for the entire population. All Danish residents are assigned a unique civil personal registration (CPR) number at birth or immigration, which allows for unambiguous linkage between registries.
We used the Danish Cancer Registry (DCR) to identify the source population of all Danish female breast cancer patients (ICD-10: C50) diagnosed between 1997 and 2011. The DCR has recorded information on all cancers diagnosed in Denmark since 1943, coded according to the International Classification of Diseases, Seventh and Tenth Revisions [13, 14]. We used the DCR to obtain information on cancer treatments received within 4 months after diagnosis. This information is available up to 2003. After 2003, we extracted information on treatment from the Danish National Patient Registry (DNPR) as described below. We also used the DCR to ascertain each patient’s date of diagnosis and cancer stage. We collected information on receptor status of the primary breast tumor through linkage with the Danish Pathology Registry, which has recorded pathology information nationwide since 1997 [15]. In Denmark, routine estrogen receptor (ER) testing was implemented from 1999 onwards; progesterone receptor (PR) testing was implemented from approximately 2006, but stopped again in 2011; human epidermal growth factor receptor 2 (HER2) testing was implemented from approximately 2007. Thus, not all patients were routinely tested in the study period. Patients with unknown receptor status were included in all analyses in a separate category. We also used records from the DNPR on non-psychiatric hospitalizations since 1977 and on outpatient and emergency room visits since 1995. Coding of diagnoses in the DNPR is performed by the physician in the outpatient clinic or physicians discharging the patient from a hospital admission [16]. This registry allowed us to identify the specific distant organs of overt breast cancer metastasis excluding lymph node metastasis during 1997–2011 (ICD-10 codes for lung, liver, brain, bone, and other metastasis sites are available in Supplemental Appendix Table 1).
To account for a potential survival difference between patients with distant metastases at the time of primary cancer diagnosis and recurrent distant disease [6, 8], we divided the cohort into patients with metastatic disease at primary diagnosis as recorded in the DCR (“de novo metastatic disease”) and those with distant metastasis at recurrence (“recurrent metastasis cohort”) as recorded in the DNPR. In the DCR, the Classification of Malignant Tumours (TNM) system has been used to stage cancers since 2004. Summary staging was used prior to 2004 (i.e., localized, regional, distant). On this basis, we defined distant metastases at the time of primary breast cancer diagnoses as M1 disease starting in 2004, according to the TNM classification, and as distant disease for patients diagnosed before 2004, according to summary staging. Recurrent metastatic breast cancer was defined as a record of recurrence recorded in the DNPR after a primary diagnosis of early breast cancer or breast cancer of unknown stage.
To account for potential delay in diagnostic work-up or registry lag time in recording, we defined site of metastasis based on all metastatic sites documented in the DNPR within 90 days of the metastatic breast cancer diagnosis for the de novo metastatic disease cohort and within 90 days of the diagnosis date of first metastasis for the recurrent metastasis cohort. The index date was thus defined as 90 days after de novo metastatic disease diagnosis or first metastasis after recurrence, respectively, for the two patient cohorts, excluding patients who did not survive 90 days after first metastasis diagnosis. We excluded all breast cancer patients recorded in the DCR with another primary cancer diagnosis before or on the index date (n = 1257).
The outcome was time to death. Person-time at risk was counted from the index date to death, emigration from Denmark, 5 years of follow-up, or 1 January 2013 (end of study period), whichever occurred first. Diagnostic codes used in this study are provided in Supplemental Appendix Table 1.
Definition of covariates
Diagnoses of all conditions included in the Charlson Comorbidity Index (CCI) were ascertained from ICD codes recorded in the DNPR prior to the index date. These provided a complete hospital inpatient and outpatient history for all women in the two study cohorts. Documentation of surgery, chemotherapy, radiotherapy, and hormonal therapy for the primary breast cancer received within 4 months of diagnosis was retrieved from the DCR through 2003 and from the DNPR thereafter. Patients were further characterized by presence of primary vs. recurrent metastatic disease, stage at primary diagnosis (localized, regional, distant, and unknown), type of treatment for the initial cancer, and receptor status (positive, negative, and unknown). Age on the index date was categorized for use in stratified analyses (≤59, 60–69, 70–79, and ≥80 years) and in regression models. Comorbidity was summarized by weights of conditions included in the CCI, excluding cancer, as all patients had metastasis, and translated into ordinal categories (scores of 0, 1–2, and ≥3). To account for regional variation, we categorized patients according to the level of urbanization of their residence on the index date [rural/smaller town (<128 inhabitants/km2), larger towns/smaller cities (128–673 inhabitants/km2), and the city of Copenhagen (>673 inhabitants/km2)]. Site of metastasis was defined on the index date using the following categorization: brain only, liver only, lung only, bone only, other and/or combinations of sites.
Statistical analysis
We calculated frequencies and proportions of patients with de novo metastatic disease and recurrent metastatic disease according to site of metastasis, number of metastatic sites at index date (one vs. several), age group, index-year categories, comorbidity scores, stage at primary diagnosis, treatment within 4 months for the initial cancer, receptor status, and level of urbanization (Table 1). The Kaplan–Meier method was used to generate survival curves overall and by site of metastasis.
We then calculated mortality risks at 1 year and between 1 and 5 years. We also calculated crude rates overall and by baseline characteristics. To estimate associations between site of metastasis and death overall and by baseline characteristics, we fitted a multivariable Cox proportional hazards model comparing relative mortality rates by site of first metastasis with the following covariates: age at index date, year of breast cancer diagnosis, stage at primary diagnosis, receptor status (including patients with unknown status in a separate category), type of treatment received at initial diagnosis, urbanization, and comorbidity score. We did this analysis separately for the primary and recurrent metastatic disease cohorts. Bone metastasis was used as the reference group in all tables because this comprised the largest group of one-site metastasis.
We then stratified the models by number of metastatic sites as of the index date (1 vs. > 1) and by receptor status, to account for potential differential survival by receptor status.
This study was approved by the Danish Data Protection Agency (journal number 1-16-02-08). As this registry-based study did not involve patient contact, no separate permission from the Danish Scientific Ethical Committee was required, according to Danish legislation.
Results
Among 4388 women diagnosed with metastatic breast cancer between 1997 and 2011, we identified 870 breast cancer patients with de novo metastatic disease and 3518 patients with recurrent metastasis diagnosed between 1997 and 2011. Table 1 shows the distribution of breast cancer patients according to the main analytic variables. The most common one-site metastasis was bone-only (30 %). Brain-only (10 %), liver-only (7.9 %), and lung-only (9.5 %) metastases were less frequent and 45 % had either other one-sites or combinations (Table 1). More than half of these patients with other/combination metastasis had combination of sites, while the most frequent one-site metastases in this group were pleura, skin, ovary, and retroperitoneum/peritoneum (Data not shown). The median age on the index date was slightly higher among patients with de novo metastatic disease than among women with recurrent metastasis (65 and 63 years, respectively) (Table 1).
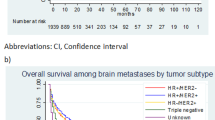
The proportion of patients with de novo metastatic disease remained relatively stable across year of breast cancer diagnosis, but proportions decreased for patients with recurrent metastatic breast cancer, likely as a consequence of the introduction of mammographic screening, advances in diagnostic work-up, and more widespread use of novel oncology drugs. The proportion of patients receiving surgery within 4 months of diagnosis for their primary cancer was lower for patients with de novo metastatic disease than for patients with recurrent disease (29 vs. 91 %), while the proportion receiving other treatments were more equal, probably reflecting broader treatment choices for patients with earlier stage breast cancer at primary diagnosis. Estrogen receptor status was available in 79 % of patients, while PR and HER2 receptor status were available in less than half during the current study period. The frequency of positive receptor status was comparable among patients with de novo metastatic disease and recurrent metastasis, as was the number of metastatic sites on the index date (Table 1). However, a larger proportion of patients in the de novo metastatic disease cohort (35 %) had bone-only metastases on the index date compared with patients with recurrent metastatic disease (26 %). In turn, a higher proportion of brain-only involvement was found among patients with recurrent metastatic disease (8.2 vs. 5.7 %). The cohort of patients with de novo metastatic disease was followed for a median of 1.3 years (interquartile range (IQR) 0.5, 2.7 years), while the cohort with recurrent disease was followed for a median of 1.5 years (IQR 0.6, 3.4 years). Figure 1 shows overall 5-year survival by metastatic site.
Mortality within 1 year of the index date
Thirty-six percent of all metastatic breast cancer patients died during the first year of follow-up. Patients with brain involvement only had the highest mortality risk (62 %) and patients with bone and lung involvement only had the lowest risk (32 % for each). Patients with brain metastases only had a more than two-fold increased risk of dying compared to patients with bone involvement only [mortality rate ratio (MRR) 2.3, 95 % confidence interval (CI) (2.0, 2.8)], adjusted for age, comorbidity, year of breast cancer diagnosis, level of urbanization, and receptor status. The MRR for liver-only metastases (1.2, 95 % CI 1.1, 1.4) was also increased, though less pronounced than for brain-only metastases. Patients with lung-only metastases (MRR 0.9, 95 % CI 0.8, 1.1) or other/combinations of metastases (MRR 1.0, 95 % CI 0.9, 1.2) had similar mortality as patients with bone metastases only. Patients with hormonal receptor-positive tumors had lower relative mortality than patients with negative receptor status (MRR 0.6, 95 % CI 0.6, 0.7). There were no marked differences in the prognostic impact of site of metastasis for primary metastasis or recurrent diseases, as shown in Fig. 2. Overall, patients with more than one metastatic site had a 30 % increased relative risk of dying in the first year after diagnosis than patients with only one metastatic site [MRR 1.3 (95 % CI 1.2, 1.5)] (data not shown). Risks and MRRs associated with specific covariates in the cohort of patients with de novo metastatic disease and the cohort of patients with recurrent metastasis are presented separately in Supplemental Appendix Table 2.
Mortality >1 to 5 years following the index date
Mortality risk between 1 and 5 years after diagnosis of metastasis was 69 %. It was highest for patients with brain-only involvement (90 %). Among patients who survived the first year after diagnosis of metastases, those with brain-only, liver-only, or other/combination of metastases continued to have higher relative mortality than patients with bone metastases only (Fig. 2; Supplemental Appendix Table 3).
Stratified analyses
Patients with metastases to sites other than bone had similar or higher mortality in all strata of receptor status. In the first year of follow-up, patients with brain metastases only had the highest MRR across receptor status strata relative to patients with bone involvement only (Table 2).
Discussion
In this large population-based cohort study, we found that the first specific organ site of metastasis is predictive of mortality in patients with metastatic breast cancer. MRRs were similar for patients with de novo metastatic disease and with recurrent distant metastasis. Patients with brain-only metastasis had the highest mortality, and patients with lung metastasis had the best prognosis. Positive hormonal receptor status on the primary tumor was a favorable prognostic factor for all metastatic sites.
Study strengths included a large sample size, a nationwide population-based design, and a setting where tax-funded universal health care is provided free of charge to all residents. Nationwide registries permitted long-term follow-up of all participants. In Denmark, breast cancer patients are treated at specialized departments following national treatment guidelines published by the Danish Breast Cancer Cooperative Group starting in 1970 [17]. Our study included all Danish patients with metastatic breast cancer recorded in the DCR and the DNPR between 1997 and 2011, except for patients who also had other primary cancer diagnoses. This eliminated the potential inclusion of metastases from other primary cancer sites. Ascertainment of primary breast cancer in the DCR is nearly complete [18].
Our study also had some limitations involving the validity of diagnoses of metastasis and potential confounding. The completeness and validity of diagnoses of metastasis recorded in the DNPR for breast cancer patients has been studied only for bone metastases, yielding a sensitivity of 0.32 (95 % CI 0.13, 0.57) and specificity of 0.99 (95 % CI 0.93, 1.00) in 100 patients from the North Denmark Region using medical records as the reference standard [19]. Our study therefore may not include all patients with recurrent breast cancer. Under-ascertainment of metastases potentially also could have had an impact on the number and sites of metastasis included in this study, as non-symptomatic metastases are unlikely to be recorded in the DNPR. Also, the threshold for using imaging to detect metastases may differ by site. Brain imaging is not a part of the staging procedure and will thus most often be performed because of symptoms while bone scintigraphy may be part of the staging procedure. As well, mortality rates may have declined in recent years due to advancements in detection and treatment [20]. Another concern is that lead time bias stemming from improved imagining and diagnostics over time could have affected our results. In recent years, novel agents with better penetration across the blood–brain barrier are being investigated for breast cancer patients with brain metastases [21]. Although breast cancer treatment and follow-up in Denmark were standardized during the years of patient study inclusion, use of novel treatment options, such as targeted therapy, may not have been reflected accurately in our cohort of patients early in the study period, which began in 1997. In addition, we lacked information on whether affected distant organs included single localized tumors or multiple lesions, which could affect treatment strategy and survival.
Breast tumors may favor metastasis to different organs and also may be associated with varying survival probabilities according to their different biological features [10]. We therefore stratified our analyses by metastatic site and receptor status to account for some of this heterogeneity. However, we lacked information on cause of death, therapies and medications prescribed for comorbid diseases, ethnicity, and other potential confounding factors. As well, receptor status was obtained from a nationwide pathology registry with incomplete recording during early study years. In addition, the analysis stratified by receptor status included only patients with available test results, and we did not re-assay receptor status in tumor blocks. Thus, the sensitivity and methods for assaying receptor status may have varied over time. In addition, the receptor status of the primary tumor may not have reflected the receptor status of tumors at the metastatic site due to tumor heterogeneity and cancer progression.
Patients with de novo metastatic disease and with recurrent metastatic disease had similar mortality after diagnosis of metastases, as observed in previous research [8]. Mortality also declined during later index years. Such temporal improvement of survival has been reported in other settings [1, 22]. Recent studies suggest that biological subtypes of breast cancer may drive metastatic behavior towards specific organs [23–25]. Specifically, hormonal receptor positivity may be associated with metastasis to the bone; triple negativity may favor the lung as a site of metastasis; and HER2 positivity may often favor metastasis to the brain or liver [10, 24]. Our sample size was too small to examine mortality as a function of breast cancer subtypes based on receptor status. However, we did adjust for receptor status in multivariable analyses to account for the discordant prognostic impact of hormonal receptor subtypes. Our study demonstrated that Danish patients with brain metastasis had the highest MRR across receptor status, relative to patients with bone involvement, only in the first year of follow-up. This accords with previous research in Italy, The Netherlands, and the United States [6, 8, 10].
There are several explanations for our results. We found similar mortality associated with both primary and recurrent metastatic breast cancer, similar to other studies [8]. Primary metastatic breast cancer is rarely treated with curative intent, and recurrent metastatic disease may be treatment refractory, limiting treatment options. We also found that patients with brain metastases had the worst prognosis. This is likely because many systemic cancer therapies fail to penetrate the blood–brain barrier, and treatment options for brain metastases are particularly limited [26]. Since overall 5-year mortality among patients with limited metastatic spread is less than 80 %, patients with distant but localized metastatic burden may benefit from more aggressive treatment [27].
Site of first metastasis is a prognostic factor for metastatic breast cancer. This association was robust to adjustment for receptor status assayed in the primary breast tumor, and patients with metastases to sites other than bone had similar or higher mortality in all strata of receptor status.
Abbreviations
- CCI:
-
Charlson Comorbidity Index
- CI:
-
Confidence interval
- DCR:
-
The Danish Cancer Registry
- DNPR:
-
The Danish National Patient Registry
- ICD:
-
International Classification of Diseases
- MRR:
-
Mortality rate ratio
- TNM:
-
Classification of malignant tumours
References
Ruiterkamp J, Ernst MF, de Munck L, van der Heiden-van der Loo M, Bastiaannet E, van de Poll-Franse LV, Bosscha K, Tjan-Heijnen VC, Voogd AC (2011) Improved survival of patients with primary distant metastatic breast cancer in the period of 1995–2008. A nationwide population-based study in the Netherlands. Breast Cancer Res Treat 128:495–503
Saphner T, Tormey DC, Gray R (1996) Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 14:2738–2746
Muss HB, Case LD, Richards F II, White DR, Cooper MR, Cruz JM, Powell BL, Spurr CL, Capizzi RL (1991) Interrupted versus continuous chemotherapy in patients with metastatic breast cancer. The Piedmont Oncology Association. N Engl J Med 325:1342–1348
Norton L (1991) Metastatic breast cancer. Length and quality of life. N Engl J Med 325:1370–1371
Andre F, Slimane K, Bachelot T, Dunant A, Namer M, Barrelier A, Kabbaj O, Spano JP, Marsiglia H, Rouzier R, Delaloge S, Spielmann M (2004) Breast cancer with synchronous metastases: trends in survival during a 14-year period. J Clin Oncol 22:3302–3308
Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH (2010) Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol 21:2169–2174
de Glas NA, Bastiaannet E, de Craen AJ, van de Velde CJ, Siesling S, Liefers GJ, Portielje JE (2015) Survival of older patients with metastasised breast cancer lags behind despite evolving treatment strategies—a population-based study. Eur J Cancer 51:310–316
Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ, van de Wouw AJ, Peters FP, van Riel JM, Peters NA, de Boer M, Peer PG, Tjan-Heijnen VC (2015) Prognosis of metastatic breast cancer: are there differences between patients with de novo and recurrent metastatic breast cancer? Br J Cancer 112:1445–1451
Ho VK, Gijtenbeek JM, Brandsma D, Beerepoot LV, Sonke GS, van der Heiden-van der Loo M (2015) Survival of breast cancer patients with synchronous or metachronous central nervous system metastases. Eur J Cancer 51:2508–2516
Gerratana L, Fanotto V, Bonotto M, Bolzonello S, Minisini AM, Fasola G, Puglisi F (2015) Pattern of metastasis and outcome in patients with breast cancer. Clin Exp Metastasis 32:125–133
Solomayer EF, Diel IJ, Meyberg GC, Gollan C, Bastert G (2000) Metastatic breast cancer: clinical course, prognosis and therapy related to the first site of metastasis. Breast Cancer Res Treat 59:271–278
Frank L (2000) Epidemiology. When an entire country is a cohort. Science 287:2398–2399
Storm HH, Michelsen EV, Clemmensen IH, Pihl J (1997) The Danish Cancer Registry—history, content, quality and use. Dan Med Bull 44:535–539
Gjerstorff ML (2011) The Danish Cancer Registry. Scand J Public Health 39:42–45
Bjerregaard B, Larsen OB (2011) The Danish Pathology Register. Scand J Public Health 39:72–74
Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT (2015) The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 7:449–490
Blichert-Toft M, Christiansen P, Mouridsen HT (2008) Danish Breast Cancer Cooperative Group–DBCG: history, organization, and status of scientific achievements at 30-year anniversary. Acta Oncol 47:497–505
Jensen AR, Overgaard J, Storm HH (2002) Validity of breast cancer in the Danish Cancer Registry. A study based on clinical records from one county in Denmark. Eur J Cancer Prev 11:359–364
Jensen AO, Norgaard M, Yong M, Fryzek JP, Sorensen HT (2009) Validity of the recorded International Classification of Diseases, 10th edition diagnoses codes of bone metastases and skeletal-related events in breast and prostate cancer patients in the Danish National Registry of Patients. Clin Epidemiol 1:101–108
Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD (2012) The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol 36:237–248
Venur VA, Leone JP (2016) Targeted therapies for brain metastases from breast cancer. Int J Mol Sci. doi:10.3390/ijms17091543
Dawood S, Broglio K, Esteva FJ, Ibrahim NK, Kau SW, Islam R, Aldape KD, Yu TK, Hortobagyi GN, Gonzalez-Angulo AM (2008) Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol 19:1242–1248
Lorusso G, Ruegg C (2012) New insights into the mechanisms of organ-specific breast cancer metastasis. Semin Cancer Biol 22:226–233
Soni A, Ren Z, Hameed O, Chanda D, Morgan CJ, Siegal GP, Wei S (2015) Breast cancer subtypes predispose the site of distant metastases. Am J Clin Pathol 143:471–478
Vaz-Luis I, Lin NU, Keating NL, Barry WT, Lii H, Winer EP, Freedman RA (2015) Racial differences in outcomes for patients with metastatic breast cancer by disease subtype. Breast Cancer Res Treat 151:697–707
Lin NU, Amiri-Kordestani L, Palmieri D, Liewehr DJ, Steeg PS (2013) CNS metastases in breast cancer: old challenge, new frontiers. Clin Cancer Res 19:6404–6418
Pagani O, Senkus E, Wood W, Colleoni M, Cufer T, Kyriakides S, Costa A, Winer EP, Cardoso F, Task Force ESO-MBC (2010) International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst 102:456–463
Author contribution
HTS and AGO conceived the study idea and designed the study. HTS and UHJ established and designed the cohort. AGO reviewed the literature and directed the study-specific analyses together with HTS, which were carried out by UHJ. All authors participated in the discussion and interpretation of the results. AGO organised the writing and wrote the initial drafts. All authors critically revised the manuscript for intellectual content and approved the final version. HTS is the guarantor for the article and accepts full responsibility for the work and the conduct of the study, had access to the data, and oversaw the decision to publish.
Funding
The study was funded by the Program for Clinical Research Infrastructure (PROCRIN) established by the Lundbeck Foundation and the Novo Nordisk Foundation and administered by the Danish Regions, and by the Danish Cancer Society (Grant No. R73-A4284-13-S17). The authors confirm independence from the sponsors; the content of the article has not been influenced by the sponsors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ording, A.G., Heide-Jørgensen, U., Christiansen, C.F. et al. Site of metastasis and breast cancer mortality: a Danish nationwide registry-based cohort study. Clin Exp Metastasis 34, 93–101 (2017). https://doi.org/10.1007/s10585-016-9824-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-016-9824-8