Abstract
Vertebral compression fracture (VCF) occurs after stereotactic body radiation therapy (SBRT) for spine metastasis. Recently, single fraction radiosurgery (sfSRS) is used more frequently. The aim of this study is to determine the clinical outcome of VCF after sfSRS. Spinal instability neoplastic score (SINS) criteria were used to retrospectively score 143 consecutive vertebral segments in 79 patients treated with SRS. Follow-up MRI, pain, and neurologic assessments obtained every 3–6 months. Pain also scored at 7, 14, and 30 days after sfSRS. Follow up was 16 ± 18 months ±SD, range 3–78. Long-term radiographic control occurred in 94 % of cases. Pain improvement resulted within 7 days in 100 % of cases with severe pain and sustained long-term in 95 %. VCF occurred in 21 % of segments: 30 % were de novo VCF. The overall 1 year fracture free probability (1yFFP) was 76 %. Pre-existing VCF resulted in higher probability to progress: 1yFFP 90 versus 60 %. Symptoms presented in 6 % of cases with de novo VCF and 39 % with progressive. The former were treated with vertebral augmentation (VA), the latter with open surgery. Surgery/VA prior to SRS did not change risk of progressive VCF. Univariate but not multivariate analysis identified histology (colorectal), pre-existing VCF, and pain (severe) as significant predictors of VCF. In conclusion, sfSRS compares favourably to SBRT for radiographic and pain control with similar VCF risk. Patients with pre-existing VCF have a higher probability to progress, become symptomatic, and require surgery. These results may help discussing risk and benefits with patients undergoing sfSRS for spinal metastasis and developing new treatment algorithms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spine metastases are the most common site of bony metastases in patients with stage IV cancer and source of significant morbidity and health care cost [1, 2]. The seminal trial by Patchell et al. [3] provided Class I evidence that open surgery followed by radiation (xRT) is superior to xRT alone in treating spinal metastasis causing cord compression, starting a new era of more aggressive surgical management. However, with the subsequent introductions of advanced SBRT techniques the treatment paradigm shifted, with surgery becoming less invasive, used in preparation for adjuvant treatment [4].
SBRT is defined as “the precise delivery of highly conformal and image-guided hypofractionated external beam radiotherapy, delivered in a single or few fractions to an extracranial body target with doses at least biologically equivalent to a radical course given over a protracted conventionally fractionated schedule” [5]. Within SBRT, sfSRS has becoming more commonly used as the dose delivery in one setting offers obvious logistics and cost benefits [6]. The recent RTOG 0631 phase 2 trial showing accurate use of SRS in a cooperative setting, provided the basis for the ongoing phase 3 trial focused on pain relief and quality of life of sfSRS compared to EBXRT [7]. The upcoming results of this trial could corroborate the evidence that sfSRS is equivalent to surgery and therefore increase the use of sfSRS as upfront treatment for spinal metastases. However, the long-term risks and their clinical significance after sfSRS have not been fully described yet.
When considering therapeutic options for spine metastasis patients, spine stability needs to be assessed as instability can only be corrected by surgical intervention. Expert consensus form the Spine Oncology Study Group developed the spine instability neoplastic score (SINS), a reliable scoring system to detect spinal instability [8–10]. Whereas, the risks of surgery are well documented [11], those of SRS are not. The risk of VCF after SBRT ranged between 11 and 39 % [5, 12], albeit its clinical significance has not been fully reported. The natural history of spinal metastases without prior therapeutic intervention shows a risk of VCF ranging from 19 to 49 % [13]. The aim of this study is to assess the risk of VCF after SRS and its clinical significance. Additionally, we investigated risk factors that could predict VCF.
Materials and methods
Patient selection and inclusion/exclusion criteria
From our prospective radiosurgery data base, we retrospectively reviewed 143 consecutive vertebral segments with metastasis treated in 95 patients with single fraction SRS, at least 3 month prior to the compilation of this data set for the period November 2007–January 2014. All patients were treated based on recommendation from a multi-disciplinary tumor board, including neurosurgeons, radiation oncologists, neuro-radiologists, neuropathologists, and oncologists. All patients had histologically proven primary cancer diagnosis. Institutional Review Board (IRB) approval was obtained at the Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Treatment technique
All patients were treated with single fraction SRS using the Novalis system (Brainlab, Munich, Germany). The treatment planning involved a diagnostic spine MRI followed by CT simulation in a body immobilizer (Alpha Cradle, Smithers Medical Products, North Canton, OH, USA). Gross tumor volume (GTV), clinical target volume (CTV), organ at risk (OAR) were contoured following published methods [7] (Fig. 1).
52 yo man with stage IV renal cell carcinoma metastatic to spine involving L1/L2 neurologically intact with VAS pain score of 8/10. a Sagittal lumbar spine MRI prior to SRS showing metastatic involvement of L1 and L2 VB; b Screen shot of single fraction SRS planning. GTV, innermost, gray (magenta); CTV, light gray (orange); 18 Gy prescription dose, dark gray (red); b Dose volume histogram (DVH); c Follow-up sagittal lumbar spine MRI 6 months after SRS showing radiographic control and lack of VCF. MRI images: T2 FSE; TR 4800 ms; TE 106 ms. (Color figure online)
Radiographic and clinical assessments
Baseline MRI and CT were used to score each vertebral segment and were independently scored by a neuroradiologist (PP) and a neurosurgeon (IMG) using SINS criteria [9]. Follow up MRI were obtained at 3, 6–9, 12 months and every 6 months thereafter, unless otherwise clinically indicated.
Vertebral body height was assessed on midline sagittal T1 MRI images. Percentage of body collapse was calculated as a percentage of the average height of adjacent vertebrae. In particular, the following formula was applied: [(V1 + V3/2) − V2]/(V1 + V3/2) being V1 and V3 measures from the vertebral bodies cranial and caudal to the metastatic one (V2). For C3 lesions, C4 measures were considered twice, being C2 longitudinal diameter inconsistent with that of C3. Similarly, L4 was used twice as a reference for L5 metastases. When an adjacent level had VCF, V1 and V3 were considered as the first cranial and caudal healthy vertebral bodies. If two adjacent vertebral bodies were involved measures from the nearby healthy vertebra were used twice.
Anterior body collapse, posterior body collapse and maximal body collapse were measured for every lesion at every time point. Measures were conducted at the anterior wall, posterior wall and most collapsed point respectively. In order to reduce the possibility of sampling error, collapse rate was calculated using baseline measures as references.
Clinical assessment of baseline pain was done using a 11 point visual analogue scale (VAS: 0 = no pain; 10 = worse pain) at baseline, 7 and 14 days after sfSRS and at each follow up MRI as above reported. Pain improvement/worsening was considered a change of at east two points on VAS. Radiographic failures were censured. Peri-procedural prophylactic dexamethasone was not used in this series.
Statistical analysis
Descriptive statistics were used to assess patients’ demographics, disease characteristics, and related covariates of interest. Categorical variables were expressed as count and proportions, whereas continued variable were expressed as mean ± standard deviation (SD) or median and range.
Fracture-free probability (FFP) was assessed using Kaplan–Meier product-limit method. The log-rank test was used as a univariate analysis to compare FFP with a potential predictor of interest. A multi-variate Cox proportional hazards regression model was used to determine the joint effect of potential factors that were found significant on univariate analysis. All p values were two-sided. Results were considered significant if p < 0.05. Statistical analysis was performed using version 19 of SPSS software (IBM, Cary, NC, USA).
Results
Patients’ demographic and baseline SINS scores are summarized in Table 1. Overall, sfSRS was used as upfront treatment in 85 % of cases. In the reminder (n = 21), sfSRS was adjuvant treatment after surgery, vertebral augmentation (VA), and/or previous EBXRT. In the past 3 years, however, sfSRS was used as upfront treatment in 95 % of cases. The mean follow up was 16 ± 18 months, range 3–78; 19 palliative cases with follow up <3 months were excluded from follow-up and outcome reporting.
Pain improvement resulted within 7 days in 100 % of patients with severe pain and sustained long-term improvement in 95 %. Increase in pain medications after sfSRS was not required by any patient in this study. None of the patients with zero baseline pain experienced worse pain in follow up. Acute pain flare reported by others [14, 15] was not observed in this study. CTV ≤46 cc3 was a significant predictor of long-term pain control on univariate analysis.
Radiographic control was obtained in 94 % (116 of 124) of cases (Fig. 1). All radiographic failures underwent surgery: 50 % (4/8) of failed cases were hepatocellular carcinoma (HCC); 25 % (2/8) renal cell carcinoma (RCC) and melanoma; 63 % (5/8) were located in the lumbar spine. New neurologic deficits did not occur in patients who sustained radiographic control. Pain/paresis occurred in all radiographic failures and resolved after surgical intervention. Acute radiation toxicity or radiation myelopathy was not observed in this study.
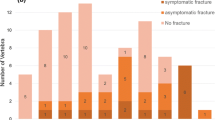
Baseline VCF was present in 42 % of cases: 8 % with VCF >50 % and 34 % with VCF ≤50 %. After sfSRS, VCF occurred in 21 % of segments, of these 30 % were de novo. The 1 year fracture free probability (FFP) was 76 %. Patients with a pre-existing VCF had a higher probability to progress, with 1 year FFP of 90 versus 60 % (Fig. 2). VCF development probability was 22 % (95 % CI 14.1, 30.5 %) at 6 months and 24 % (95 % CI 15.5, 32.7 %) at 12 months. VCF occurred within the first 6 months in 92 % of cases, with mean time to fracture of 5 months, range 3–24.
Symptoms, consisting of pain worsening, after sfSRS due to VCF occurred in 15 % of all cases; new neurologic deficits were not observed. Of note, only a minority of patients with VCF were symptomatic: 6 % of patients with de novo VCF and 39 % with progressive VCF. The former was treated with VA, the latter with open surgery (86 %) and VA (14 %). One patient with asymptomatic de novo VCF was also treated with VA. All symptoms resolved with intervention.
Univariate but not multivariate analysis identified histology (colorectal), pre-existing VCF, and pain (severe) as significant predictors of VCF (Table 2). Univariate and multivariate statistical analysis showed that surgery, even with instrumentation, does not prevent VBF, of note, however, none of these patients became symptomatic. Other SINS scores were not associated with VCF. Previously reported VCF rates in SBRT are summarized in Table 3.
Discussion
The need for therapeutic options for patients with spine metastases is increasing as the number of patients with this disease is rising [6]. Additionally, new targeted systemic therapies, for example directed toward angiogenic pathways increase the potential for compromised wound healing, therefore limiting the patient’s ability to undergo surgery as it would prolong time prior to initiating systemic therapy [16]. Data from a variety of sources show that sfSRS provides durable pain relief with quick onset after treatment and excellent local radiographic control >84 % [2, 16–20]. Since no randomized study has been done to identify the most efficacious regimen for SBRT comparing sfSRS to hypo-fractionation and to hyper-fractionation, sfSRS has become more commonly used for its logistic and cost benefit advantages [6]. This is corroborated by our study showing that sfSRS was used as the primary treatment modality in ≥84 % cases. It is therefore important to assess its potential long-term risks and clinical implications.
Delayed radiation toxicity after SBRT to esophagus, brachial and lumbar plexus has been reported and OAR dosimetric guidelines to prevent it are published [21–24]. Radiation myelopathy is another rare complication with a 1–5 % risk and with published safety guidelines to prevent it [24]. By far the most common risk after SBRT is VCF with a reported rate ranging from 11 to 39 % [5, 12]. Osteoradionecrosis is the proposed mechanisms, postulating that both vertebral bone and tumor tissue undergo replacement by friable necrotic tissue after radiation resulting in collapse [5]. However, it is important to note that VCF occurs also as part of the natural history of spine metastasis and it was reported in 19 % of patients in upfront diagnosis and 49 % at the end of the study period without any intervening treatment [13]. Similarly, in our study at baseline VCF was present in 42 % of cases.
Our data, showing a significantly increased risk of VCF in the presence of a pre-existing VCF, 1 year FFP 89 versus 60 %, raises the question of possible intervention to prevent such occurrence. Although we have a limited number of patients treated with surgery/VA prior to sfSRS, our data shows that prior surgery/VA does not result in different fracture rate. Moreover, only a minority of patients with VCF become symptomatic and required intervention, namely 6 % with de novo VCF and 39 % of progressive VCF. Thus, when discussing the risk/benefit ratio with patients undergoing sfSRS as the only therapeutic modality for spine metastases, it should be stated that the expected need for future intervention, surgery/VA, is 12 %; 6 % of treated segments (8/124) required intervention for progressive/new VCF and another 6 % required intervention for failure of tumor control.
Whereas laminectomy with or without instrumentation was the procedure of choice at the time of Patchell’s study, subsequent advances in minimally invasive techniques provide transpedicular cavitation/VA in preparation for SBRT/SRS [25–27]. Additional studies are necessary to define if the risk/benefit ratio of these procedures support their use to prevent the aforementioned 12 % of surgical need after sfSRS or if they should be used as a “planned second step” when needed.
All but one patient in our series was classified by SINS criteria as stable (19.3 %) or undetermined stability (80 %). The only unstable treated segment was a palliative case where multiple co-morbidities contra-indicated surgery and sfSRS was delivered for pain control. This reflects the effectiveness of our multi-disciplinary team approach in patients’ selection and discussion. As previously reported [4], taking into consideration the neurologic, oncologic, mechanical, and systemic (NOMS) status of each patient is essential prior to a final recommendation on treatment. Differently from previous reports [28–31] in our study, multi-variate analysis did not find SINS criteria or histology associated with increased risk of VCF, albeit univariate analysis did. One of possible explanation for this discrepancy is the homogeneity in dose delivered in our study, where 18 Gy was delivered in 87 % cases.
Conclusion
The four requirements for an effective treatment of patients with spinal metastases are pain relief, tumor control, preservation/restoration of neurologic function, and spine stability. Whereas spine stability assessed by SINS criteria can only be addressed by surgery, sfSRS should be considered as a valid option for the other three parameters. Our study corroborates the evidence that sfSRS results in sustained pain relief, tumor control, and preservation of neurologic function. Additionally, it shows that although VCF occurs in 21 % of cases after sfSRS, its long-term sequelae are limited, with surgical/VA intervention needed in only 12 % of cases. These are important aspects to consider when communicating with health care providers and patients with spinal metastatic disease in the process of choosing the best available treatment option(s).
Abbreviations
- CT:
-
Computer tomography
- CTV:
-
Clinical tumor volume
- EBRT:
-
External beam radiation
- FFP:
-
Fracture-free probability
- GTV:
-
Gross tumor volume
- HCC:
-
Hepatocellular carcinoma
- MRI:
-
Magnetic resonance imaging
- OAR:
-
Organs at risk
- RCC:
-
Renal cell carcinoma
- SBRT:
-
Stereotactic body radiation
- SINS:
-
Spine instability neoplastic score
- SRS:
-
Stereotactic radiosurgery
- sfSRS:
-
Single fraction stereotactic radiosurgery
- VA:
-
Vertebral augmentation
- VAS:
-
Visual analogue score
- VCF:
-
Vertebral compression fracture
References
Faul CM, Flickinger JC (1995) The use of radiation in the management of spinal metastases. J Neurooncol 23:149–161
Gerszten PC, Burton SA, Ozhasoglu C et al (2007) Single fraction radiosurgery for spine metastases: clinical experience in 500 cases from a single institution. Spine 32(2):193–199
Patchell R, Tibbs P, Regine W et al (2005) Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomized trial. Lancet 366:643–648
Bilsky M, Laufer I, Burch S (2009) Shifting paradigms in the treatment of metastatic spine disease. Spine 34(22):S101–S107
Sahgal A, Atenafu EG, Chao S et al (2013) Vertebral compression fracture after spine stereotactic body radiotherapy: a multi-Institutional analysis with a focus on radiation dose and spine instability neoplastic score. J Clin Oncol 31:3426–3431
Gerszten P (2014) Spine metastases: from radiotherapy, surgery, to radiosurgery. Neurosurg Online 61:16–25
Ryu S, Pugh SL, Gerszten PC et al (2014) RTOG 0631 phase 2/3 study of image guided stereotactic radiosurgery for localized (1–3) spine metastases: phase 2 results. Pract Radiat Oncol 4(2):76–81
Fisher CG, DiPaola CP, Ryken TC et al (2010) A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine 35:E1221–E1229
Fisher CG, Versteeg AL, Schouten R et al (2014) Reliability of the spinal instability neoplastic scale among radiologists: an assessment of instability secondary to spinal metastases. AJR Am J Roentgenol 203(4):869–874
Fourney DR, Frangou EM, Ryken TC et al (2011) Spinal instability neoplastic score: and analysis of reliability and validity from the Spine Oncology Study Group. J Clin Oncol 29:3072–3077
Lee BH, Park JO, Kim HS et al (2014) Perioperative complication and surgical outcome in patients with spine metastases: retrospective 200-case series in a single institute. Clin Neurol Neurosurg 122:80–86
Al-Omair A, Smith R, Kiehl TR et al (2013) A radiation-induced vertebral compression fracture following spine stereotactic radiosurgery: clinicopathological correlation. J Neurosurg Spine 18(5):430–435
Chang-Seng E, Charissoux M, Larbi A et al (2014) Spinal metastasis in breast cancer: single center experience. World Neurosurg 82(6):1344–1350
Pan HY, Allen PK, Wang XS et al (2014) Incidence and predictive factors of pain flare after spine stereotactic body radiation therapy: secondary analysis of phase 1/2 trial. Int J Radiat Oncol Biol Phys 90(4):870–876
Chiang A, Zeng L, Zhang L et al (2013) Pain flare is a common adverse event in steroid-naïve patients after spine stereotactic body radiation therapy: a prospective clinical trial. Int J Radiat Oncol Biol Phys 86(4):638–642
Aj Clark, Lambon KR, Butowski NA et al (2012) Neurosurgical management and prognosis of patients with glioblastoma that progresses during bevacizumab treatment. Neurosurgery 70:361–370
Chang EL, Shiu AS, Mendel E et al (2007) Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine 7:151–160
Gagnon GJ, Nasr NM, Liano JJ, Molzahn I, Marsh D, McRae D et al (2009) Treatment of spinal tumors using cyberknife fractionated stereotactic radiosurgery: pain and quality-of-life assessment after treatment in 200 patients. Neurosurgery 64:297–307
Ryu S, Rock J, Rosenblum M et al (2004) Patterns of failure after single-dose radiosurgery for spinal metastasis. J Neurosurg 101:402–405
Yamada Y, Bilsky MH, Lovelock DM et al (2008) High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys 71:484–490
Cox BW, Jackson A, Hunt M et al (2012) Esophageal toxicity from high-dose, single fraction paraspinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 83:e661–e662
Forquer JA, Fakiris AJ, Timmerman RD et al (2009) Brachial plexopathy from stereotactic body therapy in early stage NSCLC: dose-limiting toxicity in apical tumor sites. Radiother Oncol 93:408–413
Garg AK, Wang XS, Hiu AS et al (2011) Prospective evaluation of spinal reirradiation by using stereotactic body radiation therapy: the University of Texas MD Anderson Cancer Center experience. Cancer 117:3509–3516
Grimm J, LaCouture T, Croce R et al (2012) Dose tolerance limits and dose volume histogram evaluation for stereotactic body radiotherapy. J Appl Clin Med Phys 12:3368–3372
Gerszten P, Monaco E (2009) Complete percutaneous treatment of vertebral body tumors causing spinal compromise using transpedicular cavitation, cement augmentation, and radiosurgery. J Neurosurg 27:1–7
Gerszten P, Welch WC (2007) Combined percutaneous transpedicular tumor debulking and kyphoplasty for pathological compression fractures. Technical note. J Neurosurgical Spine 6:92–95
Rao PJ, Thayaparan GK, Fairhall JM et al (2014) Minimally invasive percutaneous fixation techniques for metastatic spinal disease. Orthop Surg 6(3):187–195
Rose PS, Laufer I, Boland PJ et al (2009) Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol 27:5075–5079
Boehling NS, Grosshans DR, Allen PK et al (2012) Vertebral compression fracture risk after stereotactic body radiotherapy for spinal metastases. J Neurosurg Spine 16(4):379–386
Cunha MVR, Al-Omair A, Atenafu EG et al (2012) Vertebral compression fracture (VCF) spine stereotactic body radiation therapy (SBRT): analysis of predictive factors. J Int Radiat Oncol Biol Phys 84:e343–e349
Sung SH, Chang UK (2014) Evaluation of risk factors for vertebral compression fracture after stereotactic radiosurgery in spinal tumor patients. Korean J Spine 11(3):103–108
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Germano, I.M., Carai, A., Pawha, P. et al. Clinical outcome of vertebral compression fracture after single fraction spine radiosurgery for spinal metastases. Clin Exp Metastasis 33, 143–149 (2016). https://doi.org/10.1007/s10585-015-9764-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-015-9764-8