Abstract
In this study, we investigated the therapeutic efficacy of EGFR2R-lytic hybrid peptide for the treatment of liver metastasis from colon carcinoma. The cytotoxic activity of the hybrid peptide against luciferase-expressing human colon cancer (HCT-116-luc) cells was determined by the WST-8 assay. The experimental mouse model of liver metastases was generated by splenic injection of HCT-116-luc cells. The hybrid peptide was intravenously injected into mice the day after cell implantation at a dose of 5 mg/kg and this was repeated on alternate days for a total of 7 doses. Saline-treated mice were used as controls. Tumor growth and therapeutic responses were monitored by an IVIS imaging system. It was shown that the hybrid peptide exhibited potent cytotoxic activity against HCT-116-luc cells and the liver metastases were significantly reduced after intravenous injections of hybrid peptide compared with controls. Furthermore, Kaplan–Meier analysis showed that hybrid peptide-treated mice had significantly longer survival than controls. In addition, bright-field and ex vivo imaging of liver tissue revealed that mice treated with the hybrid peptide had significantly fewer tumors compared with controls. These results demonstrated that the EGFR2R-lytic hybrid peptide is a potential treatment option for patients with colorectal cancer metastases in the liver.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is one of the leading causes of cancer-related deaths worldwide [1]. About 50 % of patients develop metastatic disease and the liver is the most common site of distant metastasis from colorectal cancer [2]. Surgical resection is the only potentially curative treatment option for patients with colorectal cancer metastases in the liver [3]. Surgical treatment alone for large cancers and those that have advanced into the deeper regions, still has a high failure rate [4]. During the past decades, the development of new cytotoxic drugs and regimens as well as biological targeted agents like cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor (EGFR), and bevacizumab, a monoclonal antibody targeting the vascular endothelial growth factor (VEGF), has improved the survival outcome of patients with advanced metastases [5]. These new targeted drugs expand the therapeutic arsenal for colorectal cancer to a great extent. However, the 5-year survival rates are still poor and their cost is relatively higher than conventional chemotherapy agents. Therefore, an effective therapy against liver metastasis from colon carcinoma is required.
Peptides are promising anti-tumor agents that possess high affinity and specificity, can be synthesized easily, and have a low production cost [6, 7]. We previously designed and synthesized a novel peptide that is chemically conjugated between the targeted-binding and cell-killing peptide components, and has cytotoxic and anti-tumor activities against cancer cells both in vitro and in vivo [8–12]. EGFR2R-lytic peptide is a hybrid peptide synthesized by the chemical conjugation of the targeted-binding peptide to EGFR and the cell-killing lytic peptide. It was previously suggested that the EGFR binding moiety of the hybrid peptide specifically binds to EGFR on the cell surface, and that, the lytic moiety of this peptide penetrate to make a pore and disrupts the cell membrane, leading to cell death regarding the mechanism of action of this hybrid peptide to target cells [8, 9]. In vivo analysis revealed that the EGFR2R-lytic hybrid peptide had highly potent anti-tumor activity in the EGFR-expressing human tumor xenograft model [9, 13]. EGFR is known to be highly expressed in colorectal cancer and its expression has been correlated with tumor invasion, progression, and metastasis [14]. These observations prompted us to investigate the ability of the EGFR2R-lytic hybrid peptide to prevent liver metastases from colorectal cancer.
To evaluate the therapeutic effect of the EGFR2R-lytic hybrid peptide against liver metastases from colorectal cancer in vivo, we have developed an experimental mouse model of liver metastases by splenic injection of luciferase (luc)-expressing human colon carcinoma cell HCT-116-luc, as described previously [15]. The hybrid peptide was injected into the mice via the tail vein at a dose of 5 mg/kg on alternate days for a total of 7 doses. Metastatic spread and therapeutic responses were monitored by in vivo bioluminescent imaging using the IVIS imaging system. Furthermore, the therapeutic effects of the hybrid peptide on survival in these mice were assessed and histopathological evaluations after the treatment were also performed.
Materials and Methods
Materials
The EGFR2R-lytic hybrid peptide was synthesized by the American Peptide Company (Sunnyvale, CA, USA) as described previously [9, 13]. D-luciferin potassium salt was purchased from Wako Chemicals (Osaka, Japan). The EGFR antibody was obtained from Cell Signaling Technology (Danvers, MA, USA). Other agents were mostly from Nacalai Tesque (Kyoto, Japan). All agents were of reagent-grade quality.
Cell culture
The human colon cancer cell line HCT-116 was purchased from the European Collection of Cell Culture Collection (ECACC, Salisbury, UK), and the luciferase- expressing cell line HCT-116-CMVp-luc was obtained from the Japanese Collection of Research Bioresources Cell Bank (JCRB, Osaka, Japan). The human embryonic kidney HEK293 cell line was purchased from the Health Science Research Resources Bank (HSRRB, Osaka, Japan). Cells were cultured in McCoy’s (HCT-116 and HCT-116-luc) or MEM (HEK293) containing 10 % fetal bovine serum, 100 µg/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air.
Western blotting
Western blot analyses were carried out as described previously [16]. Briefly, protein extracts were prepared from cells lysed with reporter lysis buffer (Promega, Madison, WL, USA) separated by SDS-PAGE and transferred to nitrocellulose filters using a semi-dry method. Quenched membranes were probed with antibodies and analyzed using Chemi-Lumi One Super (Nacalai Tesque, Kyoto, Japan) reagent with an LAS-3000 LuminoImage analyzer (Fujifilm, Tokyo, Japan).
Development of the human colorectal liver metastasis model
Female 7-week old BALB/c nu/nu mice (body weight, 17–20 g) were purchased from SLC Japan (Shizuoka, Japan) and maintained under specific pathogen-free conditions. All animal experiments were approved by the Animal Research Committee of Kyoto University and carried out in accordance with its guidelines. The syngeneic mouse model of human colorectal liver metastasis was developed by injecting tumor cells into the spleen, as described previously [15]. In brief, the mice were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg), and a small incision was made in the left flank to reveal the spleen. A total of 6 × 105 cells/100 µL of HCT-116-luc cells were injected into the spleen using a 27-gauge needle. Cotton swabs containing 75 % ethanol were firmly placed on the spleen injection site to prevent intraperitoneal seeding and metastasis. Three minutes after cell implantation, splenectomy was done, and the incision was sutured.
Cytotoxic activity in vitro
Cancer and normal cells were treated with various concentrations of EGFR2R-lytic hybrid peptide for 48 h at 37 °C and cell viability was measured using the WST-8 assay, as described previously [8].
In vivo tumor growth inhibition assay
To assess the anti-tumor efficacy of the EGFR2R-lytic hybrid peptide against liver metastases from colorectal cancer, the hybrid peptide was injected via the tail vein at a dose of 5 mg/kg on alternate days for a total of 7 times. Control mice were injected with saline. The growth and metastasis of the tumors were monitored by bioluminescence imaging using the IVIS imaging system. To visualize the bioluminescence signal of tumor cells, mice were injected intraperitoneally with 100 μl of 10 mg/ml D-luciferin potassium salt (dissolved in D-PBS) and the bioluminescence images were taken after 10 min.
Histopathological analysis
Mice were sacrificed 35 days after tumor cell implantation. The liver tissues were collected, fixed in 4 % para-formaldehyde, and embedded in paraffin. Sections were stained with hematoxylin and eosin (H&E).
Statistical analysis
All values were expressed as mean ± SD. The Student’s t test was used to determine whether differences between the means of two groups were significant. Differences were considered statistically significant at P < 0.05. Survival rates were assessed using the Kaplan–Meier method [17].
Results
EGFR protein expression levels and the cytotoxic activity of the EGFR2R-lytic hybrid peptide
First, we investigated the expression levels of EGFR in human colon cancer HCT-116 and luciferase-expressing HCT-116-luc cells by western blot analysis. As shown in Fig. 1a, HCT-116-luc cells showed moderate expression of EGFR, and the expression levels were similar to that of non-transfected cells HCT-116, which is consistent with previous reports [18, 19]. HEK293 cells expressing a low level of EGFR were used as the negative control and β-actin was used as the internal control. The relative protein expression levels of EGFR are shown in Fig. 1b. We further investigated the cytotoxic activity of the EGFR2R-lytic hybrid peptide against HCT-116 and HCT-116-luc cells in vitro using the WST-8 assay. HEK293, HCT-116, and HCT-116-luc cells were seeded into a 96- well plate at a density of 3000 cells per well and treated with increasing concentrations of EGFR2R-lytic hybrid peptide for 48 h. The results are shown in Fig. 1c. EGFR2R-lytic hybrid peptide showed potent cytotoxic activities against both HCT-116 and HCT-116-luc cells in a concentration-dependent manner, and there was no significant difference between the two cell lines. HEK293 cells were used as control. The half maximal inhibitory concentrations (IC50) were 12.7 ± 3.2 and 11.3 ± 1.2 µM for HCT-116 and HCT-116-luc cells, respectively (Table 1). It was confirmed that both cell types displayed similar sensitivities to the EGFR2R-lytic hybrid peptide, and cytotoxic activity correlated with their expression level of EGFR, which were the same. Taken together, these results demonstrated that the EGFR2R-lytic hybrid peptide has effective cytotoxic activity against EGFR-expressing human colorectal cancer cells HCT-116-luc in vitro.
In vitro cytotoxic activity of the EGFR2R-lytic hybrid peptide against EGFR-expressing cancer cells. a Western blot analysis of the expression of EGFR protein in human colon cancer HCT-116 and luciferase-expressing HCT-116-luc cells. HEK293 cells were used as the negative control and β-actin was used as the internal control. b Relative EGFR expression normalized to β-actin. c Cytotoxic activities of the EGFR2R-lytic hybrid peptide against HCT-116 and HTC-116-luc cells. The experiments were repeated three times and similar results were obtained. Data are expressed as percentages of the untreated control cells (100 % of control) and as mean ± SD from triplicate determinations
Therapeutic effects of the EGFR2R-lytic hybrid peptide on liver metastasis from colorectal cancer in vivo
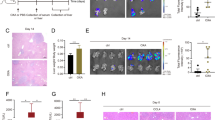
Next, to evaluate the therapeutic efficacy of the EGFR2R-lytic hybrid peptide against liver metastasis in vivo, we developed an experimental mouse model of liver metastases by injecting HCT-116-luc cells into the spleen (Fig. 2a). A total of 6 × 105 cells/100 µL of human HCT-116-luc cells were surgically implanted into the spleen of nude mice to induce intrasplenic tumor growth. Then, to investigate the fate of tumor cells in the microcirculation after implantation, bioluminescence signals of HCT-116-luc cells were visualized following an intraperitoneal injection of D-luciferin (50 mg/kg) and imaging was performed using the IVIS imaging system. As shown in Fig. 2b, strong bioluminescence signals were detected in the liver of mice after tumor cell implantation, but signals were not detected in other regions. It was confirmed that the tumor cells injected into the spleen passed almost immediately through the portal circulation into the liver and can form colonies. The EGFR2R-lytic hybrid peptide was intravenously injected the day after tumor cell implantation (5 mg/kg on alternate days for a total of 7 doses) and the therapeutic responses in the liver metastasis mouse model were monitored sequentially every 10 days. Whole-body imaging is shown in Fig. 3. The mean relative luminescence count in the liver of EGFR2R-lytic hybrid peptide-treated mice was significantly lower than that of saline-treated control mice at all time points. Moreover, the bright field and ex vivo imaging showed that the mice treated with the EGFR2R-lytic hybrid peptide had significantly fewer metastases and lower tumor burden compared with saline-treated control mice 20 days after the last treatment (Fig. 4a and b). The liver tissue from non-tumor-bearing mice was used as the negative control. Furthermore, the histological examination showed multiple, hypercellular, solid tumor lesions in the liver of saline-treated control mice, while small metastatic lesions were detected in mice that were intravenously injected with the EGFR2R-lytic hybrid peptide (Fig. 4c). None of the mice died of hybrid peptide-induced toxicity and no other significant adverse events were observed during the experiments. These results indicated that the EGFR2R-lytic hybrid peptide could potentially inhibit tumor growth and metastasis in the mouse model of EGFR-expressing human colon carcinoma in vivo.
Development and confirmation of the model of liver metastases from colorectal cancer. a Schematic representation of the experimental protocol. A total of 6 × 105 cells/100 µL of HCT-116-luc cells were injected into the spleen using a 27-gauge needle and 3 min after cell implantation, splenectomy was done, and the incision was sutured. b Bioluminescence imaging on day 1 post–intrasplenic injection of HCT-116-luc cells. Mice were injected intraperitoneally with 100 µL of 10 mg/mL D-luciferin potassium salt, and 10 min afterwards, the bioluminescence images were taken using the IVIS imaging system
Therapeutic effect of the EGFR2R-lytic hybrid peptide in the mouse model of liver metastases from colorectal cancer. a In vivo living imaging. Mice were treated intravenously with or without 5 mg/kg EGFR2R-hybrid peptide from day 2 post—intrasplenic injection of HCT116-luc cells for a total of seven doses. b Relative bioluminescence intensity. Data are expressed as mean ± SD for 4–5 mice/group
Therapeutic effect of the EGFR2R-lytic hybrid peptide on liver tissues in the mouse model of liver metastases from colorectal cancer on day 30 post–intrasplenic injection of HCT-116-luc cells. a Bright-field imaging of metastatic lesions in liver tissues from non-tumor-bearing mice (negative control, n = 2), and saline- and EGFR2R-lytic hybrid peptide-treated mice (n = 3/group). The dotted lines show the means of the number of metastasis-positive liver tissues of each group. b Ex vivo imaging of excised liver tissues using the IVIS imaging system. c Hematoxylin and eosin (H&E) staining of liver sections from each treatment group. N normal, T tumor. Scale bars 200 µm
Prolonged survival effects of the EGFR2R-lytic hybrid peptide for the liver metastasis mouse model in vivo
We further investigated the therapeutic effects of the EGFR2R-lytic hybrid peptide on survival in the liver metastasis mouse model using Kaplan–Meier analysis. As shown in Fig. 5a, the mice treated with the EGFR2R-lytic hybrid peptide exhibited a median survival of more than 100 days compared with 60 days in the saline-treated control group. As shown above, we found that EGFR2R-lytic hybrid peptide had cytotoxic activity on HCT-116-luc cells and showed anti-tumor activity on human colon carcinoma xenografts. Based on these results, we speculated that the EGFR2R-lytic hybrid peptide reduced the growth of tumor cells, leading to prolonged survival of the tumor-bearing mice. There were no significant differences between saline- and EGFR2R-lytic hybrid peptide-treated mice in body weight (Fig. 5b). These results demonstrated that the EGFR2R-lytic hybrid peptide could significantly prolong the survival of mice, without inducing toxicity.
Kaplan–Meier analysis of survival curves and body weights of mice with liver metastases from colorectal cancer after treatment with the EGFR2R-lytic hybrid peptide. a Survival curves. b Body weights. Data are expressed as mean ± SD for 10 mice/group. Student’s t test was used for analyzing the differences between the two treatment groups. *P < 0.05, compared with saline-treated group
It is known that metastatic liver cancer is the most common fatal liver disease, and liver function tests examining the levels of blood serum markers, liver enzymes, and certain proteins are conducted to investigate parameters that are linked to or affected by cancer. In this study, biochemical and hematological analyses were performed at 24 h or 20 days after the last treatment of EGFR2R-lytic hybrid peptide. The biochemical and hematological tests showed that the aspartate transaminase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) were higher in both saline- and EGFR2R-lytic hybrid peptide-treated mice with liver metastases compared with the negative control, non-tumor-bearing mice (Supplementary Table 1). AST, ALT, and LDH were significantly higher in saline-treated control mice than in EGFR2R-lytic hybrid peptide-treated mice at both 24 h and 20 days after the last treatment of EGFR2R-lytic hybrid peptide. It was previously reported that the AST and ALT levels were significantly higher in patients with liver metastasis than in those without liver metastasis [20]. In particular, LDH was increased in patients with liver metastasis and correlated with the survival time of colorectal cancer patients who had an increased risk of liver metastasis [20]. Therefore, elevated AST, ALT, and LDH indicate that HCT-116-luc colon cancer cells injected into the spleen had spread to the liver and induced liver damage, and these results demonstrate that the EGFR2R-lytic hybrid peptide-treated mice had significantly less liver damage than saline-treated control mice.
Discussion
We previously reported that the EGFR2R-lytic hybrid peptide had cytotoxic activity toward cancer cells expressing EGFR and anti-tumor activity in subcutaneous MDA-MB-231 breast cancer with K-ras mutation xenograft model [9]. Here, we investigated whether the EGFR2R-lytic hybrid peptide could prevent the outgrowth of liver metastasis from human colon carcinoma. First, to develop an optimal model of liver metastasis, we selected the HCT-116 cell as the target cell model for evaluation of therapeutic efficacy of the EGFR2R-lytic hybrid peptide because of their EGFR expression level and suitability for the development of the liver metastasis model [21, 22]. The HCT-116-luc cells that stably express firefly luciferase were used in this study for monitoring tumor growth by the IVIS imaging system.
Western blot analysis showed that the HCT-116-luc cells expressed levels of EGFR that were almost the same as that of non-transfected parental HCT-116 cells (Fig. 1a). Additionally, the WST-8 assay showed that the EGFR2R-lytic hybrid peptide had potent cytotoxic activities against both HCT-116-luc and HCT-116 cells with IC50 values of 11.3 ± 1.2 and 12.7 ± 3.2 µM, respectively (Table 1). There was no significant difference between the sensitivities of the two cell lines to the EGFR2R-lytic hybrid peptide. These results confirmed that the HCT-116-luc cell was just as appropriate as parental HCT-116 cells for developing the EGFR-expressing colorectal cancer model and the EGFR2R-lytic hybrid peptide is capable of inhibiting the growth of HCT-116-luc cells in vitro. In contrast to the HCT-116-luc cells, HEK293 cells expressed very low levels of EGFR, and exhibited much weaker sensitivity to the EGFR2R-lytic hybrid peptide, which is consistent with previous results [9].
The rate of metastasis formation presumably depends on a large number of factors, including the type of primary tumor cells, the number of tumor implants, and the cell implantation route [23–26]. Among them, the implantation route is closely related to death and metastatic distribution of tumor cells in mice. Kryriazis et al. [23] reported that human colorectal carcinoma cell lines injected into the peritoneum of nude mice produced lung metastases, whereas the same cells grown subcutaneously did not result in metastases. In this study, the in vivo liver metastasis mouse model was developed by intrasplenic injection of 6 × 105/100 µL HCT-116-luc cells followed by splenectomy. Intrasplenic injection of tumor cells has long been known as an effective method of developing liver metastases in nude mice, whereas portal vein injection of tumor cells can result in the rapid death of tumor cells [22, 27, 28]. Hoffman and co-workers [29–31] reported that orthotopic implantation was a more faithful model of clinically observed liver metastases. However, orthotopic implantation results in varying degrees of metastasis among the animals and involves a long experimental duration of several months. In contrast, the model that was established in this study by intrasplenic injection of HCT-116-luc cells has a relatively high reproducibility and takes only a short time to reach the experimental endpoint. On the one hand, intrasplenic tumor cell injection results in rapid colonization of tumor cells not only in the liver but also in the spleen. The major tumor burden is within the spleen, leading to shorter survival in mice (data not shown). The higher tumor burden in the spleen also influences the anti-tumor activity of the EGFR2R-lytic hybrid peptide against liver metastases. Hence, we carried out splenectomy after intrasplenic injection of HCT-116-luc tumor cells into nude mice in this study.
Bioluminescence imaging showed that even as soon as one day after HCT-116-luc tumor cell injection followed by splenectomy, a clear signal from the tumor cells was detected in the liver (Fig. 2b). It was shown that the HCT-116-luc cells injected into the spleen could migrate directly into the liver through the portal circulation. However, the bioluminescence signal intensity was much weaker at 10 days after tumor cell implantation than the initial signal of bioluminescence cells within the livers at day 1. It is possible that the unattached tumor cells undergo apoptosis, and the attached tumor cells survive and begin to proliferate and form metastasis. In vivo results showed that the intravenously injected EGFR2R-lytic hybrid peptide significantly inhibited the tumor growth of HCT-116-luc cells and the development of metastases. We previously reported that the 1 mg/kg EGFR2R-lytic hybrid peptide significantly inhibit tumor growth in a subcutaneous tumor model [9]. In this study, the EGFR2R-lytic hybrid peptide dose was set at 5 mg/kg, given on alternate days for a total of 7 times. This treatment strategy may be more clinically applicable than the GRGDS peptide, which is a pentapeptide sequence that appears to be critical for cell interaction with fibronectin and high doses (3 mg) of the peptide are required to obtain a sufficient anti-metastatic effect [32, 33]. The dose of the EGFR2R-lytic hybrid peptide is relatively higher in comparison with other anti-cancer drugs since it has a short circulatory half-life [13]. However, our previous results indicated that the EGFR2R-lytic hybrid peptide at 5 mg/kg had no effect on total body weight, and no abnormality was apparent on histological examination of various tissues and hematological analysis [13]. In addition, in this study, the levels of AST, ALT, and LDH were significantly increased in saline-treated mice that had liver metastases, while EGFR2R-lytic hybrid peptide-treated groups of mice showed no significant differences compared with non-tumor-bearing mice (Supplementary Table 1), indicating that the EGFR2R-lytic hybrid peptide dose at 5 mg/kg did not show any treatment–related abnormalities in terms of hematological and biochemical parameters. We also determined the median lethal dose of this hybrid peptide in healthy mice in vivo. BALB/c mice were injected intravenously with a single dose of EGFR2R-lytic hybrid peptide at 10, 15 and 20 mg/kg, respectively. There was no lethal toxicity in the mice, which were administrated with 10 mg/kg. Although, 2 of 5 mice, which were administrated with 15 mg/kg died, the significant toxicity was not observed in the survived three mice. Contrary, all five mice, which were administrated with 20 mg/kg died after the injection. Thus, the lethal dose in 50 % was estimated between 15 and 20 mg/kg (Supplementary Table 2). Thus, based on previous studies and the present results, it was demonstrated that the EGFR2R-lytic hybrid peptide at 5 mg/kg is effective at preventing tumor growth, without causing toxicity.
Furthermore, significant prolonged survival was observed in mice with liver metastases from colorectal cancer that had EGFR2R-lytic hybrid peptide treatment (median survival after saline or EGFR2R-lytic hybrid peptide treatment: 60 vs. >100 days, respectively; P < 0.05). The survival time of EGFR2R-lytic hybrid peptide-treated mice with liver metastases from colorectal cancer suggests that the EGFR2R-lytic hybrid peptide is effective at preventing the formation of metastases, resulting in not only inhibition of colony formation, but also substantial prolongation of life-span.
Colorectal cancer has remarkably high metastaticity and the liver is the most common site for metastasis of colorectal cancer because the venous drainage of the colon is through the portal vein, which goes directly to the liver [1–3]. The prognosis of patients with liver metastases from colorectal cancer is very poor. Also, the K-ras mutations detected in colorectal cancer confer resistance to tyrosine kinase (TKI)-targeted therapy [34, 35]. The HCT116 cells used in this study is a K-ras mutant cell line and we previously reported that the EGFR2R-lytic hybrid peptide had potent cytotoxic and anti-tumor activities against TKI-resistant cancer cells with K-ras mutations [8, 9]. The results of the current study and previous reports, indicate that intravenous administration of EGFR2R-lytic hybrid peptide is able to significantly reduce the formation of HCT-116 colon carcinoma–derived metastases within the liver and prolong the survival of mice. Thus, the EGFR2R-lytic hybrid peptide is a potentially effective agent for the treatment of patients with liver metastases from colorectal cancer.
Abbreviations
- EGFR:
-
Epidermal growth factor receptor
- VEGF:
-
Vascular endothelial growth factor
- PBS:
-
Phosphate buffered saline
- Luc:
-
Luciferase
- H&E:
-
Hematoxylin and eosin
- AST:
-
Aspartate transaminase
- ALT:
-
Alanine aminotransferase
- LDH:
-
Lactate dehydrogenase
- TKI:
-
Tyrosine kinase
References
Hawk ET, Linburg PJ, Viner JL (2002) Epidemiology and prevention of colorectal cancer. Surg Clin N Am 82(5):905–941
Patanaphan V, Salazar OM (1993) Colorectal cancer: metastatic patterns and prognosis. South Med J 86(1):38–41
Scheele J, Stangl R, Altendorf-Hofmann A (1990) Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg 77(11):1241–1246
Clark ME, Smith RR (2014) Liver-directed therapies in metastatic colorectal cancer. J Gastrointest Oncol 5(5):374–387
Tol J, Punt CJ (2010) Monoclonal antibodies in the treatment of metastatic colorectal cancer: a review. Clin Ther 32(3):437–453
Reubi JC, Maecke HR (2008) Peptide-based probes for cancer imaging. J Nucl Med 49(11):1735–1738
Thundimadathil J (2012) Cancer treatment using peptides: current therapies and future prospects. J. Amino Acids 2012:967347
Kohno M, Horibe T, Haramoto M, Yano Y, Ohara K, Nakajima O, Matsuzaki K, Kawakami K (2011) A novel hybrid peptide targeting EGFR-expressing cancers. Eur J Cancer 47(5):773–783
Tada N, Horibe T, Haramoto M, Ohara K, Kohno M, Kawakami K (2011) A single replacement of histidine to arginine in EGFR-lytic hybrid peptide demonstrates the improved anticancer activity. Biochem Biophys Res Commun 407(2):383–388
Kawamoto M, Horibe T, Kohno M, Kawakami K (2011) A novel transferrin receptor-targeted hybrid peptide disintegrates cancer cell membrane to induce rapid killing of cancer cells. BMC Cancer 11:359–372
Yang L, Horibe T, Kohno M, Haramoto M, Ohara K, Puri RK, Kawakami K (2012) Targeting interleukin-4 receptor α with hybrid peptide for effective cancer therapy. Mol Cancer Ther 11(1):235–243
Horibe T, Kawamoto M, Kohno M, Kawakami K (2012) Cytotoxic activity to acute myeloid leukemia cells by Antp-TPR hybrid peptide targeting Hsp90. J Biosci Bioeng 114(1):96–103
Gaowa A, Horibe T, Kohno M, Sato K, Harada H, Hiraoka M, Tabata Y, Kawakami K (2014) Combination of hybrid peptide with biodegradable gelatin hydrogel for controlled release and enhancement of anti-tumor activity in vivo. J Control Release 176:1–7
Amador ML, Hidalgo M (2004) Epidermal growth factor receptor as a therapeutic target for the treatment of colorectal cancer. Clin Colorectal Cancer 4(1):51–62
Otten MA, van der Bij GJ, Verbeek SJ, Nimmerjahn F, Ravetch JV, Beelen RH, van de Winkel JG, van Egmond M (2008) Experimental antibody therapy of liver metastases reveals functional redundancy between Fc gammaRI and Fc gammaRIV. J Immunol 181(10):6829–6836
Kohno M, Horibe T, Ohara K, Ito S, Kawakami K (2014) The membrane-lytic peptides K8L9 and melittin enter cancer cells via receptor endocytosis following subcytotoxic exposure. Chem Biol 21(11):1522–1532
Haramoto M, Kohno M, Nakajima O, Horibe T, Kiyohara M, Fukazawa H, Togawa T, Kawakami K (2010) Pancreatic cancer therapy with a novel pump for controlled drug release. Oncol Rep 23(2):365–370
Xu H, Yu Y, Marciniak D, Rishi AK, Sarkar FH, Kucuk O, Majumdar AP (2005) Epidermal growth factor receptor (EGFR)-related protein inhibits multiple members of the EGFR family in colon and breast cancer cells. Mol Cancer Ther 4(3):435–442
Balin-Gauthier D, Delord JP, Rochaix P, Mallard V, Thomas F, Hennebelle I, Bugat R, Canal P, Allal C (2006) In vivo and in vitro antitumor activity of oxaliplatin in combination with cetuximab in human colorectal tumor cell lines expressing different level of EGFR. Cancer Chemother Pharmacol 57(6):709–718
Wu XZ, Ma F, Wang XL (2010) Serological diagnostic factors for liver metastasis in patients with colorectal cancer. World J Gastroenterol 16(32):4084–4088
Rajput A, Dominguez San Martin I, Rose R, Beko A, Levea C, Sharratt E, Mazurchuk R, Hoffman RM, Brattain MG, Wang J (2008) Characterization of HCT116 human colon cancer cells in an orthotopic model. J Surg Res 147(2):276–281
Ishizu K, Sunose N, Yamazaki K, Tsuruo T, Sadahiro S, Makuuchi H, Yamori T (2007) Development and characterization of a model of liver metastasis using human colon cancer HCT-116 cells. Biol Pharm Bull 30(9):1779–1783
Kyriazis AP, DiPersio L, Michael GJ, Pesce AJ, Stinnett JD (1978) Growth patterns and metastatic behavior of human tumors growing in athymic mice. Cancer Res 38(10):3186–3190
Kozlowski JM, Fidler IJ, Campbell D, Xu ZL, Kaighn ME, Hart IR (1984) Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer Res 44(8):3522–3529
Giavazzi R, Jessup JM, Campbell DE, Walker SM, Fidler IJ (1986) Experimental nude mouse model of human colorectal cancer liver metastases. J Natl Cancer Inst 77(6):1303–1308
Suemizu H, Monnai M, Ohnishi Y, Ito M, Tamaoki N, Nakamura M (2007) Identification of a key molecular regulator of liver metastasis in human pancreatic carcinoma using a novel quantitative model of metastasis in NOD/SCID/gammacnull (NOG) mice. Int J Oncol 31(4):741–751
Fidler IJ (1990) Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res 50(19):6130–6138
Bouvet M, Tsuji K, Yang M, Jiang P, Moossa AR, Hoffman RM (2006) In vivo color-coded imaging of the interaction of colon cancer cells and splenocytes in the formation of liver metastases. Cancer Res 66(23):11293–11297
Fu XY, Besterman JM, Monosov A, Hoffman RM (1991) Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci USA 88(20):9345–9349
Wang X, Fu X, Hoffman RM (1992) A new patient-like metastatic model of human lung cancer constructed orthotopically with intact tissue via thoracotomy in immunodeficient mice. Int J Cancer 51(6):992–995
An Z, Jiang P, Wang X, Moossa AR, Hoffman RM (1999) Development of a high metastatic orthotopic model of human renal cell carcinoma in nude mice: benefits of fragment implantation compared to cell-suspension injection. Clin Exp Metastasis 17(3):265–270
Humphries MJ, Olden K, Yamada KM (1986) A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science 233(4762):467–470
Komazawa H, Saiki I, Igarashi Y, Azuma I (1993) Inhibition of tumor metastasis by a synthetic polymer containing a cell-adhesive RGDS peptide. J Bioact Compat Polym 8:258–274
Schimanski CC, Linnemann U, Berger MR (1999) Sensitive detection of K-ras mutations augments diagnosis of colorectal cancer metastases in the liver. Cancer Res 59(20):5169–5175
Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359(17):1757–1765
Acknowledgments
We thank Mitsuko Tachi, Yoshie Masuda and Nanako Okushima (Department of Pharmacoepidemiology, Kyoto University) for their technical assistance in cell culture and animal studies. This study was supported by Grants-in-Aid for Young Scientist (A) (Grant No. 23680089) from the Japan Society for the Promotion of Science. This study was also supported in part by a collaboration research fund from Olympus Corporation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Koji Kawakami serves as a scientific advisor to Olympus Corporation. None of the other authors have any potential competing interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gaowa, A., Horibe, T., Kohno, M. et al. Potent anti-tumor effects of EGFR-targeted hybrid peptide on mice bearing liver metastases. Clin Exp Metastasis 33, 87–95 (2016). https://doi.org/10.1007/s10585-015-9760-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-015-9760-z