Abstract
B chromosomes are dispensable elements that occur in many species, including maize. The maize B chromosome is acrocentric and highly heterochromatic and undergoes nondisjunction during the second pollen mitosis. In this study, we determined the genetic behavior and organization of two naturally occurring B chromosome variants (designated Bta and Btb). The morphology and genetic behavior of the Bta chromosome were similar to those of the typical B chromosome, but the Bta chromosome contained a deletion in the first heterochromatin region and had higher transmission frequencies through both male and female parents. The Btb chromosome was reduced in size, consisted primarily of heterochromatin, and had a lower transmission frequency. The Btb chromosome lacked nondisjunctional behavior, which was restored by the presence of normal B chromosomes in the cell. Furthermore, the Btb chromosome contained two centromeric regions, only one of which was active. The organization of these two naturally occurring B chromosome variants was also determined using fluorescence in situ hybridization with B-associated sequences and by amplification of B-specific molecular markers to create possible evolutionary models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Supernumerary B chromosomes are dispensable chromosomes that contain large selfish DNA elements. B chromosomes were first described more than a century ago (Wilson 1907) and have been identified in approximately 15 % of eukaryotic species (Jones and Rees 1982; Camacho 2005). Generally, B chromosomes do not pair or recombine with any of the normal chromosomes (A chromosomes) during meiosis, are often heterochromatic, lack detectable genetic effects on individuals, and are transmitted in a non-Mendelian manner (Jones et al. 2008). Although the genetic features and molecular compositions of B chromosomes have been revealed, their origin and evolution remain a mystery (Jones and Houben 2003; Jones et al. 2008; Houben et al. 2014). Due to their nonessential nature, B chromosome polymorphisms are expected among populations. Indeed, several B chromosome structural variants have been identified in plants such as Brachycome dichromosomatica (Houben et al. 1999), Scilla autumnalis (Parker et al. 1991), and the rye Secale cereale (Marques et al. 2012), as well as in animals such as the grasshopper Eyprepocnemis plorans (Bakkali et al. 1999). These studies indicate that most B chromosome variants have a monophyletic origin from a unique type of ancestral B chromosome.
The maize B chromosome was identified a century ago (Kuwada 1915). During the pachytene stage of meiotic prophase I, the typical B chromosome contains a diminutive short arm and a long arm with a heterochromatic knob adjacent to the centromere, a proximal euchromatic region, a large block of distal heterochromatin, and a distal euchromatic region (McClintock 1933). To ensure its survival, the maize B chromosome has evolved several mechanisms, such as nondisjunction during the second pollen mitosis (Longley 1927; Roman 1947) and preferential fertilization of the egg by sperm carrying B chromosomes (Roman 1948). The nondisjunction mechanism requires trans-acting elements located in the proximal and distal euchromatic regions of the B chromosome long arm (Ward 1973; Lin 1978). To obtain insight into the nature and origin of the maize B chromosome, numerous molecular approaches have been applied to evaluate the organization and transcription of its DNA. The results have led researchers to isolate repetitive elements and molecular markers specific to the maize B chromosome (Alfenito and Birchler 1993; Stark et al. 1996; Lin and Chou 1997; Cheng and Lin 2004; Peng et al. 2005; Lamb et al. 2007; Chien et al. 2014; Lin et al. 2014; Kao et al. 2015). However, most of the identified maize B chromosomal DNA is similar to A chromosomal DNA (Chilton and McCarthy 1973; Alfenito and Birchler 1993; Stark et al. 1996; Cheng and Lin 2003; Lamb et al. 2005; Lo et al. 2009).
In maize, the genetic behavior and mitotic structure of four types of B chromosome variants derived from the typical B chromosome have been documented (Randolph 1941). However, a detailed model of the possible origin of these variants has not been proposed. Moreover, a collection of small maize B chromosomes that vary in size was identified during the breakage-fusion-bridge (BFB) cycle (McClintock 1939, 1941) of a translocation between the B chromosome and the short arm of chromosome 9 (Zheng et al. 1999). The structure and transmission rates of these B chromosome variants, as well as their pairing, disjunction and sister chromatid cohesion during meiosis, have been reported (Kato et al. 2005; Han et al. 2007). Furthermore, engineered minichromosomes were constructed by modifying the maize B chromosome using telomere sequence-mediated chromosome truncation and were characterized (Yu et al. 2007; Masonbrink et al. 2013). These minichromosomes offer enormous potential for the development of artificial chromosomes for use in plant breeding and biotechnology (Birchler and Han 2013; Houben et al. 2013).
To gain greater understanding of the origin of the naturally occurring B chromosome variants, we identified and characterized two structural B chromosome variants derived from the typical maize B chromosome. We found that both B chromosome variants displayed different transmission characteristics compared with those of the typical B chromosome, and that one of them lost its nondisjunctional ability. Using fluorescence in situ hybridization with B-associated sequences and amplification assays of B-specific molecular markers, the organization of these two B chromosome variants was determined, and accordingly, possible models of their origin were proposed.
Materials and methods
Plant materials
The maize inbred line L289, which carries typical B chromosomes, was propagated by crossing euploid L289 plants in our laboratory for decades. Two progeny plants containing B chromosome variants (designated Bta and Btb) that differ in morphology from that of the typical B chromosome were identified and characterized. The Bta chromosome was discovered in a progeny plant derived from an L289 plant containing two B chromosomes, and the Btb chromosome was discovered in a progeny plant derived from an L289 plant containing one B chromosome.
Cytogenetic procedures
Chromosomal constitutions were determined in Feulgen-stained root-tip cells as described by Lin (1977). To determine the nondisjunction and transmission frequencies of the B variants, L289 plants with one B, Bta, or Btb chromosome were crossed as males and females with euploid L289 plants. To test the nondisjunction of the Btb chromosome in the presence of the normal B chromosome, an L289 plant carrying two B and one Btb chromosomes was crossed as the male to a euploid L289 plant. The chromosomal constitutions of the resulting progeny were identified in root-tip cells. Chromosomes at different meiotic stages were prepared in pollen mother cells from L289 plants bearing two B, Bta, or Btb chromosomes following the standard protocol (Cheng and Lin 2003). The lengths of the pachytene chromosomes were measured using an imaging system (DP2-BSW ver. 2.1, Olympus Corp., Tokyo, Japan).
Fluorescence in situ hybridization (FISH) was performed according to the method of Cheng (2010). The B-repeat probe specific to the B chromosome centromere was generated using the clone B1.1a (Cheng 2010). The CL-repeat probe was generated using the clone pCLa1 (Cheng and Lin 2004). The Stark repeat probe, generated using the clone pStark-1, was provided by J. A. Birchler (Lamb et al. 2007). The CentC probe was derived from clone CC0B-1 (Peng and Cheng 2011). The 180-bp knob repeat was derived from clone pBPC21 (Cheng and Lin 2003). The probe directed against the telomere was cloned as described in Wang and Chen (2003), and the probe directed against the long terminal repeats of the maize retroelement CentA was cloned as described in Mroczek and Dawe (2003), except that a pGEM-T easy vector (Promega, Madison, WI, USA) was used. The identity of the DNA insert was confirmed by sequencing. The fluorescence signals were captured using a cooled charge-coupled device camera (DP73, Olympus Corp.) on an Olympus BX51 fluorescence microscope and were processed using Photoshop (Adobe, San Jose, CA, USA).
Genomic DNA isolation and B-specific molecular marker amplification
Maize genomic DNA was isolated according to Lin and Chou (1997). Primers for six maize B-specific molecular markers were used, including CL-repeat (Cheng and Lin 2004), Stark (Lamb et al. 2007), SCAR313, SCAR345, SCAR349, and SCAR426 (Kao et al. 2015). PCR was performed in a 25-μl reaction mixture containing 10 mM Tris-HCl, 50 mM KCl, 1.5 mM MgCl2, l M betaine (Sigma-Aldrich, MO, USA), 100 μM of each dNTP, 0.25 μM of each primer (see Supplemental Table 1), 100 ng of genomic DNA, and 0.5 units of Pro-Taq DNA polymerase (Protech, Taipei, Taiwan). Amplification was performed in a Perkin Elmer GeneAmp 2400 Thermal Cycler under the following conditions: 5 min at 94 °C; 30 cycles of 30 s at 94 °C; 30 s at the annealing temperature (Supplemental Table 1) and 90 s at 72 °C; and a final extension for 10 min at 72 °C. The resulting products were separated via 1 % agarose gel electrophoresis. A pair of maize actin gene-specific amplification primers was used to produce an internal control, according to the method of Kao et al. (2015).
Results
Chromosomal structures of B chromosome variants
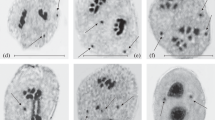
During mitotic metaphase, the morphology of the Bta chromosome was similar to that of the typical B chromosome (Fig. 1a, b), but the Btb chromosome was reduced in size (Fig. 1c). According to FISH using probes directed against the centromere-specific sequence CentC (Ananiev et al. 1998) and the B-centromere-specific sequence B-repeat (Alfenito and Birchler 1993), both the B and Bta chromosomes exhibited B-repeat signals adjacent to the CentC signals at one end, as well as CentC signals along the long arm (Fig. 1d, e). However, the Btb chromosome displayed centromere-specific signals at both ends, suggesting it was a dicentric chromosome (Fig. 1f). The presence of B-repeat signals confirmed that the two B variants were derived from the typical B chromosome. To determine the fine structures of the B variants, bivalent chromosomes were observed during the pachytene stage. The pachytene B chromosome consists of a short arm, a centromeric knob (CK), proximal euchromatin (PE), four blocks of distal heterochromatin (DH1–DH4), and distal euchromatin (DE) (Fig. 1g). The pachytene Bta chromosome was similar to the B chromosome but contained a smaller first heterochromatin region (DH1’) than that of the B chromosome (Fig. 1h). The ratio between lengths of the DH1 region and the B chromosome was 0.08 ± 0.1 (n = 10), and that of the DH1’ region and the Bta chromosome was 0.05 ± 0.1 (n = 10; Supplemental Table 2). In contrast, the pachytene Btb chromosome only carried four blocks of heterochromatin (H1–H4) (Fig. 1i).
Chromosomal structures of B chromosome variants. Mitotic metaphase cells containing the typical B (a, d), Bta (b, e), or Btb (c, f) chromosome. Arrows indicate the B chromosome and B variants. The maize centromere-specific CentC probe (green) and the B-chromosome-specific B-repeat probe (red) were used to detect the B and A chromosome centromeres (d–f). The pachytene B chromosome consists of a short arm (S), a centromeric knob (CK), proximal euchromatin (PE), four blocks of distal heterochromatin (DH1–DH4), and distal euchromatin (DE) (g). The pachytene Bta chromosome is similar to the B chromosome but contains a block of distal heterochromatin (DH1’) that is smaller than the DH1 block of the B chromosome (h). The pachytene Btb chromosome consists of four blocks of heterochromatin (H1–H4) (i). Scale bars are all equal to 10 μm
Homolog pairing, transmission, and nondisjunction of B chromosome variants
To determine the behavior of the B chromosome variants during cell division, mitotic cells containing a single B, Bta, or Btb chromosome were hybridized with the B-repeat and CentC probes. The results showed that all three B chromosome types moved normally during metaphase, anaphase, and telophase, suggesting that each of the B variants had a functional centromere (Supplemental Figure S1). During the diakinesis of pollen mother cells carrying two B, Bta, or Btb chromosomes, 100 % (n = 81) of the observed cells containing the typical B chromosome formed bivalents, whereas 10 % (n = 131) of those containing the Bta chromosome and 62 % (n = 148) of those containing the Btb chromosome formed two unpaired univalents (Supplemental Table 3 and Fig. 2). These results support a positive correlation between chromosome size and homolog pairing, as described by Han et al. (2007).
The transmission frequencies of L289 plants carrying one B variant chromosome through their male and female gametes were 31.6 and 25 % for the B chromosome, 41.7 and 43.4 % for the Bta chromosome, and 22.4 and 20 % for the Btb chromosome, respectively (Table 1). The nondisjunction frequencies of the B and Bta chromosomes during the second pollen mitosis were determined to be 54 and 57.2 %, respectively. The Btb chromosome lacked the ability to undergo nondisjunction. However, when normal B chromosomes were also present, nondisjunction of the Btb chromosome was restored (Table 2). This result was expected, given that the PE and DE regions of the B chromosome are essential for nondisjunction (Ward 1973; Lin 1978). Moreover, novel structural B variants that differed in size and morphology from the Bta or Btb chromosome were observed in seven progeny (Tables 1 and 2). The two progenies of plants carrying the Bta chromosome contained B variants that were smaller than the Bta chromosome, and the four progenies of plants carrying the Btb chromosome contained B variants that were larger than the Btb chromosome. The remaining one B variant derived from the Btb chromosome was a metacentric chromosome (data not shown).
Distribution of maize repetitive elements on B chromosome variants
To investigate the organization of the B chromosome variants in detail, maize repetitive elements that have been localized to the B chromosome, including the B-repeat, telomere repeat, 180-bp knob repeat, CL-repeat, Stark repeat, CentC, and CentA (Lamb et al. 2005), were used as FISH probes for hybridization of pachytene bivalents of the B variants (Fig. 3). B-repeat signals were observed at the distal half of the CK on the B and Bta chromosomes (Fig. 3a, b) but were detected at both terminals of the Btb chromosome (Fig. 3c). Telomere signals were detected at both distal ends of all three chromosomes (Fig. 3a–c). The 180-bp knob repeat signals were found at the proximal half of the CK of the B and Bta chromosomes (Fig. 3d, e). On the Btb chromosome, the 180-bp knob repeat signals were present near the B-repeat signals at the distal end of the H4 block (Fig. 3f). The CL-repeat probe hybridized to the first three heterochromatin regions of the B and Bta chromosomes (Fig. 3g, h) and to all four heterochromatin regions of the Btb chromosome (Fig. 3i). The Stark repeat was detected specifically at the DH3 block of the B and Bta chromosomes (Fig. 3j, k) and at the H3 block of the Btb chromosome (Fig. 3i). CentC signals were observed in multiple regions along the length of the B and Bta chromosomes, including a CK region that colocalized with a region of intense B-repeat signaling and the four blocks of heterochromatin (Fig. 3m, n). On the Btb chromosome, the CentC signals colocalized with the B-repeat signals at the distal half of the H1 block and were located at the proximal half of the H1 block, as well as at the other three blocks of heterochromatin (Fig. 3o). The CentA signals on the B and Bta chromosomes were located at the CK near the B-repeat signals and at the four heterochromatin regions (Fig. 3p, q). On the Btb chromosome, CentA signals were detected at the proximal half of the H1 block as well as at the other three blocks of heterochromatin (Fig. 3r). The above FISH results are graphically presented in Fig. 4.
FISH analysis of B chromosome variants. Pachytene bivalents of the B, Bta, and Btb chromosomes were hybridized with FISH probes specific for various maize repetitive elements (green), including a telomere repeat (a–c), 180-bp knob repeat (d–f), CL-repeat (g–i), Stark repeat (j–l), CentC repeat (m–o), and CentA repeat (p–r). Chromosomes were stained with DAPI (blue) and labeled with the B-repeat probe (red). Scale bars are all equal to 10 μm
Hybridization patterns of maize repetitive elements on B chromosome variants. The diagrams illustrate the hybridization patterns of probes for various repetitive elements on the B (a), Bta (b), and Btb (c) chromosomes. The pachytene B chromosome consists of a short arm (S), a centromeric knob (CK), proximal euchromatin (PE), four blocks of distal heterochromatin (DH1–DH4), and distal euchromatin (DE). The pachytene Bta chromosome is similar to the B chromosome but contains a first block of distal heterochromatin (DH1’) that is smaller than the DH1 block of the B chromosome. The pachytene Btb chromosome carries four blocks of heterochromatin (H1–H4). Arrows indicate the position of the active centromere
The active centromere of B chromosome variants
To determine the position of the active centromere on B variants, we used the B-repeat and the 180-bp knob repeat as FISH probes to analyze the B variants during meiotic metaphase I. On the B and Bta chromosomes, the 180-bp knob repeat signals were located behind the B-repeat signals at the most poleward positions (Fig. 5a, b), indicating that the CK regions of the B and Bta chromosomes contained active centromeres (Fig. 4a, b). On the dicentric Btb chromosome, a sole B-repeat signal was observed at the most poleward position, and another B-repeat signal that colocalized with the 180-bp knob repeat signals was observed at the equatorial plate (Fig. 5c). Accordingly, the active centromere of the dicentric Btb chromosome was located in the distal half of the H1 region, whereas the centromere at the end of the H4 region was inactivated (Figs. 3f and 4c).
Determination of the functional centromere of B chromosome variants. Meiotic metaphase I cells containing bivalent B (a), Bta (b), or Btb (c) chromosomes were hybridized with the 180-bp knob repeat (green) and the B-repeat (red) probes. Chromosomes were stained with DAPI (blue). White arrows indicate the B-repeat signals at the most poleward positions. Scale bars are all equal to 10 μm
Amplification of B-specific molecular markers of B chromosome variants
To determine the fine structural rearrangements that occurred on the B variants, primer pairs that amplify six B-specific markers that have been mapped to definitive B chromosome regions were used for PCR of the genomic DNA of plants carrying the three B chromosome types. As shown in Table 3 and Supplemental Figure S2, the CL-repeat marker was located at the CK and the first three heterochromatin regions of the B chromosome (Cheng 2010). The Stark marker was mapped to the DH3 region of the B chromosome (Lamb et al. 2007). The four B-specific sequence-characterized amplified region (SCAR) markers were located throughout the four distal heterochromatin regions of the B chromosome, as follows: SCAR426 at DH1; SCAR313 at DH1 and DH2; SCAR345 at DH1 and DH3; and SCAR349 at DH1 and DH4 (Kao et al. 2015). Genomic DNA from L289 plants lacking the B chromosome (0B) and containing one B (1B), Bta, or Btb chromosome was used as the template to amplify the six B-specific molecular markers. As shown in Table 3 and Supplemental Figure S3, PCR products corresponding to each marker were present in the 1B genomic DNA but were absent in the 0B genomic DNA, supporting the B chromosome specificity of these markers. Identical amplification patterns of the CL-repeat and Stark markers were obtained using 1B, Bta, and Btb genomic DNA. PCR for the four SCAR markers yielded similar amplification patterns, with lower levels of the products produced using Bta genomic DNA as the template and only the SCAR345 product obtained using Btb genomic DNA as the template.
Discussion
Few studies have been published regarding the naturally occurring B chromosome derivatives in maize. Randolph (1941) described the four types of B chromosome derivatives, called the C, D, E, and F chromosomes, which are progressively smaller fragments of the typical B chromosome. The D chromosome, which resembles the Btb chromosome, is spherical, with a diameter roughly equivalent to that of an ordinary chromosome. Longley (1956) proposed that these chromosomes originated from a foldback of a univalent B chromosome occurring during mid-prophase of meiosis, as described by McClintock (1933). The foldback configuration may have been due to homologous pairing between the different regions of the B chromosome, with subsequent exchanges in these regions leading to the formation of diminutive B chromosomes.
Based on the results of this study and those of Lamb et al. (2005), repetitive-element sequences are abundant in the CK and the four distal heterochromatin regions of the B chromosome (Figs. 3 and 4); these repetitive elements provide the foundation for homologous pairing between the different regions of the B chromosome. Accordingly, we can propose several possible processes that may have shaped the formation of the Bta and Btb chromosomes during meiosis. The Bta chromosome may have arisen from an unequal crossing over in the DH1 region of the B chromosome (Fig. 6a). Based on the smaller size of the DH1’ region in the Bta chromosome (Supplemental Table 2 and Fig. 1h) and the reduced level of amplification of PCR products representing the four DH1-localized SCAR markers obtained using Bta genomic DNA (Table 3 and Supplemental Figure S3), a single unequal crossing over may have occurred between the DH1 regions of two homologous B chromosomes, resulting in the formation of the DH1’ region of the Bta chromosome and the deletion of the distal portion of the DH1 region, the site of the four B-specific SCAR markers.
Possible modes of origin of the Bta and Btb chromosomes. The typical B chromosome consists of a short arm (S), a centromeric knob (CK), proximal euchromatin (PE), four blocks of distal heterochromatin (DH1–DH4), and distal euchromatin (DE). a A Bta chromosome carrying a smaller DH1’ region can be generated via an unequal crossing over (black lines) between the DH1 regions of two homologous B chromosomes during meiosis. b Dyscentric pairing of a univalent B chromosome followed by U-shaped exchanges (black lines) between the CK and DH2 regions and between the CK and DH4 regions, and misdivision (dashed line) of the centromere will produce a dicentric chromosome, two telocentric chromosomes, a ring chromosome, and an acentric fragment. Then, the loss of function of one centromere, amplification of heterochromatin, and addition of telomeric repeats on the dicentric chromosome will produce a Btb chromosome containing four blocks of heterochromatin (H1–H4). Red boxes indicate regions of B-repeats, and gray boxes indicate regions of 180-bp knob repeats. Euchromatin regions are indicated with gray lines, and heterochromatin regions are indicated with black boxes
The formation of the Btb chromosome from the B chromosome is more complicated. Longley (1956) proposed that the dyscentric pairing of a univalent B chromosome with an X-shaped or U-shaped exchange produced the diminutive B chromosome. Therefore, a univalent B chromosome could pair dyscentrically, followed by exchanges between different chromosomal regions and misdivision of the centromere during meiosis I to generate a dicentric chromosome. This dicentric chromosome may subsequently suffer the loss of function of one centromere, the amplification of heterochromatin, and the addition of telomeric repeats to produce the Btb chromosome (Fig. 6b). The Btb chromosome apparently lacks the short arm, possibly due to the misdivision of the centromere of a univalent B chromosome during meiosis I, which would result in the formation of telocentric chromosomes, as described by Kaszás and Birchler (1996). The broken ends were healed via the addition of telomeric repeats to stabilize this chromosome (Fig. 3c).
Furthermore, FISH analysis showed that the H1 region of the Btb chromosome exhibited B-repeat signals that colocalized with CentC signals at the distal half and signals for three repetitive elements, CL-repeat, CentC, and CentA, at the proximal half (Fig. 4c). This signal organization in the H1 region could be derived from a U-shaped exchange between the CentC sequences at the centromeric region of the CK and the proximal half of the DH2 region in the dyscentric pairing configuration of the B chromosome that caused the loss of the CK, PE, DH1, and partial DH2 fragments from the dicentric chromosome (Fig. 6b), as shown by the absence of the 180-bp knob repeat at the H1 region of the Btb chromosome (Figs. 3f and 4c) and the absence of the three SCAR markers in the DH1 or DH2 regions in the Btb genomic DNA (Table 3 and Supplemental Figure S3). Similarly, another U-shaped exchange could occur between the 180-bp knob sequences at the CK and the DH4 region (Lamb et al. 2005) that would result in the loss of the DE and of partial DH4 fragments from the dicentric chromosome (Fig. 6b). This possibility was supported by the absence of SCAR349 from the DH4 region in the Btb genomic DNA (Table 3 and Supplemental Figure S3) and the loss of nondisjunction, which requires the DE of the B chromosome (Ward 1973), by the Btb chromosome (Table 1). The exchange also led to the reduction of the 180-bp knob repeats at the H4 region of the Btb chromosome (Fig. 3f).
To be stable, a dicentric chromosome must have one active and one inactive centromere; otherwise, two active centromeres will lead to chromosome breakage during cell division. In maize, several stable dicentric chromosomes have been identified based on the B-A translocation of chromosomes undergoing the chromosome type BFB cycle (Han et al. 2006; Liu et al. 2015). In the dicentric Btb chromosome, the centromere in the distal half of the H1 region is active, but the centromere at the end of the H4 region has become inactive (Figs. 4c and 5c). CentC repeats are the key elements of maize centromeres (Jin et al. 2004), and the functional centromere of the B chromosome is a small, CentC-rich domain that is embedded with a large array of B - repeats (Jin et al. 2005). Thus, it is rational to conclude that the centromere in the distal half of the H1 region is active because CentC signals colocalized with the B - repeat in this region but not at the end of the H4 region (Figs. 3o and 4c).
Heterochromatin amplification has been proposed to occur in chromosomes in in vitro plant cultures (Lapitan et al. 1984; Johnson et al. 1987) and during the evolution of B chromosomes in Plantago lagopus (Dhar et al. 2002) and maize (Cheng 2010). Subsequently, heterochromatin amplification may occur on the dicentric chromosome to form the Btb chromosome, which carries four blocks of heterochromatin (Fig. 6b). The H3 fragments on the Btb chromosome were predominantly derived from the DH3 region of the B chromosome based on the observation of the DH3-specific Stark signals at the H3 region of the Btb chromosome (Fig. 3l). Fragments of the other three heterochromatic blocks found on the Btb chromosome may be derived from the distal portion of the DH2 region, the proximal portion of the DH4 region, or the portion of the DH3 region that lacks the Stark sequences.
Several distinct properties have been documented in the Gramineae by which B chromosomes enhance their transmission and accumulate in nature (Jones 1995). In rye, the B chromosomes are maintained in populations through nondisjunction during the first pollen mitosis and the first egg cell mitosis and by then directing themselves in an unreduced number into the sperm and egg nuclei (Houben et al. 2014). The frequency of nondisjunction in pollen is consistently high, and a trans-acting signal controlling nondisjunction has been mapped to the distal part of the long arm of rye B chromosomes (Lima-De-Faria 1962). In maize, the B chromosome ensures its own survival by nondisjunction during the second pollen mitosis, together with the preferential fertilization of the egg by the sperm carrying the B chromosomes (Longley 1927; Roman 1948) which result in a higher frequency of transmission through the male parent (Table 1; Randolph 1941). In our study, the nondisjunction frequency of the Bta chromosomes was similar to that of the typical B chromosome. However, interestingly, the male and female transmission frequencies of this chromosome were similar, and both were higher than that of the typical B chromosome (Table 1). The deletion in the DH1’ region or other undetectable rearrangements on the Bta chromosome should account for its unusual transmission characteristics. The Btb chromosome lost the ability for nondisjunction due to the lack of PE and DE regions, which contain trans-acting elements essential for nondisjunction of the B chromosome (Ward 1973; Lin 1978). However, if a typical B chromosome is present in a cell containing a Btb chromosome, nondisjunction of both types of chromosome occurs (Table 2). Furthermore, the small Btb chromosome showed lower transmission frequencies through both parents, comparable to the transmission behavior of mini B chromosomes generated by other means (Kato et al. 2005; Yu et al. 2007).
Several de novo structural B chromosome variants were observed in the progeny of L289 plants carrying the Bta or Btb chromosome (Tables 1 and 2). These variant chromosomes differed in size and morphology from the Bta and Btb chromosomes, and they might have been generated via scenarios similar to those described above (Fig. 6). In maize, minichromosomes generated by transgene-mediated telomere seeding in the B chromosome (Yu et al. 2007) and BFB cycles of a translocated B chromosome (Zheng et al. 1999) have been analyzed and could be used to develop chromosome-based vector systems (Kato et al. 2005; Han et al. 2007; Birchler and Han 2013; Houben et al. 2013; Masonbrink et al. 2013; Graham et al. 2015). Structural B variants derived from naturally occurring Bta or Btb chromosomes have the potential to function as new sources of minichromosomes for the development of plant artificial chromosomes in the future.
Abbreviations
- BFB:
-
Breakage-fusion-bridge
- CK:
-
Centromeric knob
- DE:
-
Distal euchromatin
- DH:
-
Distal heterochromatin
- FISH:
-
Fluorescence in situ hybridization
- PE:
-
Proximal euchromatin
- SCAR:
-
Sequence-characterized amplified region
References
Alfenito MR, Birchler JA (1993) Molecular characterization of a maize B chromosome centric sequence. Genetics 135:589–597
Ananiev EV, Phillips RL, Rines HW (1998) Chromosome-specific molecular organization of maize (Zea mays L.) centromeric regions. Proc Natl Acad Sci USA 95:13073–13078
Bakkali M, Cabrero J, Lopez-Leon MD, Perfectti F, Camacho JP (1999) The B chromosome polymorphism of the grasshopper Eyprepocnemis plorans in North Africa. I. B variants and frequency. Heredity 83:428–434
Birchler JA, Han F (2013) Meiotic behavior of small chromosomes in maize. Front Plant Sci 4:505
Camacho JPM (2005) B chromosome. In: Gregory TR (ed) The evolution of the genome. Elsevier, San Diego, pp 223–286
Carlson WR (1969) Factors affecting preferential fertilization in maize. Genetics 62:543–554
Cheng YM (2010) Evolution of the heterochromatic regions on maize B long arm based on the sequence structure of CL-repeat variants. Chromosome Res 18:605–619
Cheng YM, Lin BY (2003) Cloning and characterization of maize B chromosome sequences derived from microdissection. Genetics 164:299–310
Cheng YM, Lin BY (2004) Molecular organization of large fragments of maize B chromosome: indication of a novel repeat. Genetics 166:1947–1961
Chien YL, Lin CY, Lo KL, Cheng YM (2014) Development and mapping of CL-repeat display markers on the maize B chromosome. Cytogenet Genome Res 144:227–236
Chilton MD, McCarthy BJ (1973) DNA from maize with and without B chromosomes: a comparative study. Genetics 74:605–614
Dhar MK, Friebe B, Koul AK, Gill BS (2002) Origin of an apparent B chromosome by mutation, chromosome fragmentation and specific DNA sequence amplification. Chromosoma 111:332–340
Graham ND, Cody JP, Swyers NC, McCaw ME, Zhao C, Birchler JA (2015) Engineered minichromosomes in plants: structure, function, and applications. Int Rev Cell Mol Biol 318:63–119
Han F, Lamb JC, Birchler JA (2006) High frequency of centromere inactivation resulting in stable dicentric chromosomes of maize. Proc Natl Acad Sci USA 103:3238–3243
Han F, Gao Z, Yu W, Birchler JA (2007) Minichromosome analysis of chromosome pairing, disjunction, and sister chromatid cohesion in maize. Plant Cell 19:3853–3863
Houben A, Thompson N, Ahne R, Leach CR, Verlin D, Timmis JN (1999) A monophyletic origin of the B chromosomes of Brachycome dichromosomatica (Asteraceae). Plant Syst Evol 219:127–135
Houben A, Mette MF, Teo CH, Lermontova I, Schubert I (2013) Engineered plant minichromosomes. Int J Dev Biol 57:651–657
Houben A, Banaei-Moghaddam AM, Klemme S, Timmis JN (2014) Evolution and biology of supernumerary B chromosomes. Cell Mol Life Sci 71:467–478
Jin W, Melo JR, Nagaki K et al (2004) Maize centromeres: organization and functional adaptation in the genetic background of oat. Plant Cell 16:571–581
Jin W, Lamb JC, Vega JM, Dawe RK, Birchler JA, Jiang J (2005) Molecular and functional dissection of the maize B chromosome centromere. Plant Cell 17:1412–1423
Johnson SS, Phillips RL, Rines HW (1987) Possible role of heterochromatin in chromosome breakage induced by tissue culture in oats (Avena sativa L.). Genome 29:439–446
Jones RN (1995) B chromosomes in plants. New Phytol 131:411–434
Jones N, Houben A (2003) B chromosomes in plants: escapees from the A chromosome genome? Trends Plant Sci 8:417–423
Jones RN, Rees H (1982) B chromosomes. Academic, London
Jones RN, Viegas W, Houben A (2008) A century of B chromosomes in plants: so what? Ann Bot 101:767–775
Kao KW, Lin CY, Peng SF, Cheng YM (2015) Characterization of four B-chromosome-specific RAPDs and the development of SCAR markers on the maize B-chromosome. Mol Genet Genomics 290:431–441
Kaszás E, Birchler JA (1996) Misdivision analysis of centromere structure in maize. EMBO J 15:5246–5255
Kato A, Zheng YZ, Auger DL et al (2005) Minichromosomes derived from the B chromosome of maize. Cytogenet Genome Res 109:156–165
Kuwada Y (1915) Ueber die Chromosomenzahl von Zea mays L. Bot Mag Tokyo 29:83–89
Lamb JC, Kato A, Birchler JA (2005) Sequences associated with A chromosome centromeres are present throughout the maize B chromosome. Chromosoma 113:337–349
Lamb JC, Riddle NC, Cheng YM, Theuri J, Birchler JA (2007) Localization and transcription of a retrotransposon-derived element on the maize B chromosome. Chromosome Res 15:383–398
Lapitan NL, Sears RG, Gill BS (1984) Translocations and other karyotypic structural changes in wheat x rye hybrids regenerated from tissue culture. Theor Appl Genet 68:547–554
Lima-De-Faria A (1962) Genetic interaction in rye expressed at the chromosome phenotype. Genetics 47:1455–1462
Lin BY (1977) A squash technique for studying the cytology of maize endosperm and other tissues. Stain Technol 52:197–201
Lin BY (1978) Regional control of nondisjunction of the B chromosome in maize. Genetics 90:613–627
Lin BY, Chou HP (1997) Physical mapping of four RAPDs in the B chromosome of maize. Theor Appl Genet 94:534–538
Lin HZ, Lin WD, Lin CY, Peng SF, Cheng YM (2014) Characterization of maize B-chromosome-related transcripts isolated via cDNA-AFLP. Chromosoma 123:597–607
Liu Y, Su H, Pang J et al (2015) Sequential de novo centromere formation and inactivation on a chromosomal fragment in maize. Proc Natl Acad Sci USA 112:E1263–E1271
Lo KL, Lin YP, Chen LJ, Lin BY (2009) Isolation and characterization of new maize B sequences from a microdissected library. Plant Mol Biol Report 27:350–354
Longley AE (1927) Supernumerary chromosomes in Zea mays. J Agric Res 35:769–784
Longley AE (1956) The origin of diminutive B-type chromosomes in maize. Am J Bot 43:18–22
Marques A, Klemme S, Guerra M, Houben A (2012) Cytomolecular characterization of de novo formed rye B chromosome variants. Mol Cytogenet 5:34
Masonbrink RE, Fu S, Han F, Birchler JA (2013) Heritable loss of replication control of a minichromosome derived from the B chromosome of maize. Genetics 193:77–84
McClintock B (1933) The association of non-homologous parts of chromosomes in the mid-prophase of meiosis in Zea mays. Z Zellforsch Mikrosk Anat 19:191–237
McClintock B (1939) The behavior in successive nuclear divisions of a chromosome broken at meiosis. Proc Natl Acad Sci USA 25:405–416
McClintock B (1941) The stability of broken ends of chromosomes in Zea mays. Genetics 26:234–282
Mroczek RJ, Dawe RK (2003) Distribution of retroelements in centromeres and neocentromeres of maize. Genetics 165:809–819
Parker JS, Lozano R, Taylor S, Rejón MR (1991) Chromosomal structure of populations of Scilla autumnalis in the Iberian Peninsula. Heredity 67:287–297
Peng SF, Cheng YM (2011) Characterization of satellite CentC repeats from heterochromatic regions on the long arm of maize B-chromosome. Chromosome Res 19:183–191
Peng SF, Lin YP, Lin BY (2005) Characterization of AFLP sequences from regions of maize B chromosome defined by 12 B-10L translocations. Genetics 169:375–388
Randolph LF (1941) Genetic characteristics of the B chromosome in maize. Genetics 26:608–631
Roman H (1947) Mitotic nondisjunction in the case of interchanges involving the B-type chromosome in maize. Genetics 32:391–490
Roman H (1948) Directed fertilization in maize. Proc Natl Acad Sci USA 34:36–42
Stark EA, Connerton I, Bennett ST, Barnes SR, Parker JS, Forster JW (1996) Molecular analysis of the structure of the maize B-chromosome. Chromosome Res 4:15–23
Wang CJ, Chen CC (2003) Localization of centromere and telomere sequences on maize pachytene chromosomes by fluorescence in situ hybridization. BioFormosa 38:17–25
Ward EJ (1973) Nondisjunction: localization of the controlling site in the maize B chromosome. Genetics 73:387–391
Wilson EB (1907) The supernumerary chromosomes of Hemiptera. Science 26:870–871
Yu W, Han F, Gao Z, Vega JM, Birchler JA (2007) Construction and behavior of engineered minichromosomes in maize. Proc Natl Acad Sci USA 104:8924–8929
Zheng YZ, Roseman RR, Carlson WR (1999) Time course study of the chromosome-type breakage-fusion-bridge cycle in maize. Genetics 153:1435–1444
Acknowledgments
This study was supported by grants from the National Science Council grant of Taiwan (NSC 102-2311-B-005-001) and the Ministry of Science and Technology of Taiwan (MOST 104-2311-B-005-012-MY3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jiming Jiang.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. S1
Mitotic behavior of B chromosome variants. Cells in mitotic metaphase (a, d, g), anaphase (b, e, h), and telophase (c, f, i) carrying one copy of the B (a-c), Bta (d-f), or Btb (g-i) chromosome were hybridized with the CentC (green) and the B-repeat (red) probes. Chromosomes were stained with DAPI (blue). White arrows indicate the B chromosome or B variants. Scale bars all equal to 10 μm (GIF 95 kb)
Supplemental Fig. S2
Locations of six B-specific molecular markers on the B chromosome. The figure was modified from Kao et al. (2015) and shows where four B-specific SCAR markers (SCAR313, SCAR345, SCAR349 and SCAR426) were mapped using the breakpoints of 15 B-A translocations (Arrows). The locations of the CL-repeat and Stark markers were indicated based on the results of Cheng (2010) and Lamb et al. (2007), respectively. Black lines indicate the mapped positions of the six B-specific markers. Dotted lines represent regions in which the presence of the markers cannot be verified. S, short arm; CK, centromeric knob; PE, proximal euchromatin; DH1-DH4, four blocks of distal heterochromatin; DE, distal euchromatin (GIF 24 kb)
Supplemental Fig. S3
Amplification of six B-specific molecular markers from B chromosome variants. Genomic DNA from L289 plants lacking the B chromosome (0B) and containing one B (1B), Bta, or Btb chromosome was amplified using primers for the B-specific markers CL-repeat, Stark, SCAR426, SCAR313, SCAR345 and SCAR349. Quality and an equal quantity of the genomic DNA of different genotypes were conducted by amplification using a maize Actin primer pair. M molecular weight marker (molecular weights are shown at the left) (GIF 188 kb)
Supplemental Table 1
(DOCX 56 kb)
Supplemental Table 2
(DOCX 56 kb)
Supplemental Table 3
(DOCX 54 kb)
Rights and permissions
About this article
Cite this article
Cheng, YM., Feng, YR., Lin, YP. et al. Cytomolecular characterization and origin of de novo formed maize B chromosome variants. Chromosome Res 24, 183–195 (2016). https://doi.org/10.1007/s10577-015-9516-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-015-9516-2