Abstract
Recent evidences have shown the therapeutic potential of transcranial photobiomodulation on traumatic brain injury and Alzheimer’s disease. Despite the promising benefits in the brain, little is known about the laser’s effects in the absence of pathological conditions. We submitted young (4 months old) and aged (20 months old) rats to transcranial low-level laser and evaluated their exploratory activity and habituation in open field, anxiety in elevated plus maze, spatial memory in Barnes maze, and aversive memory in a step-down inhibitory avoidance task. Additionally, the levels of a panel of inflammatory cytokines and chemokines were quantified in two different brain regions: the cerebral cortex and the hippocampus. Young and aged rats submitted to transcranial laser exhibited better cognitive performance in Barnes maze than did control rats. Transcranial laser therapy decreased cortical levels of GM-CSF, IL-10, MCP-1, LIX, and TNFα in young rats and IL-5 in aged rats. High levels of IL-6, IL-10, and TNF-alpha were found in the cerebral cortex of aged rats submitted to transcranial laser. In the hippocampus, a decrease in IP-10 and fractalkine levels was observed in the aged rats from the laser group when compared to the aged rats from the control group. Our data indicate that transcranial photobiomodulation improves spatial learning and memory and alters the neuroinflammatory profile of young and aged rats’ brains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is a progressive multifactorial process associated with cognitive function impairment and increased susceptibility to neurodegenerative diseases (Mattson and Magnus 2006). During aging, immune function is attenuated (immunosenescence) (Castle 2000) and the activation of immune system cells triggers a more reactive phenotype, increasing the activation of neuroinflammatory cytokines (Godbout et al. 2005). Interestingly, an imbalance between pro- and anti-inflammatory cytokines has been observed in the aged brain with a shift toward a pro-inflammatory state (Godbout and Johnson 2009).
Currently, several non-pharmacological treatments have been proposed for the improvement of cognitive functions in the elderly, such as cognitive therapy (Olazaran et al. 2004; Rozzini et al. 2007) and physical exercise (Cardoso et al. 2017). Photobiomodulation therapy (PBMT) using lasers and LEDs has attracted the interest of the scientific community for being a non-invasive therapy, that may have the capability of promoting several beneficial effects, such as faster healing of lesions and reduced sensitivity to pain and inflammation in several diseases (Bjordal et al. 2006a, b; Chung et al. 2012). For instance, Albertini et al. (2004) first verified that laser therapy was effective in reducing inflammation in rat paw edema similar to the classical NSAID diclofenac. Lopes-Martins et al. (2005) noted that laser therapy was able to reduce carrageenan-induced mice pleurisy and Bjordal et al. (2006b) observed that the laser inhibited prostaglandin E2 production in patients with achilles tendinitis using a real-time microdialysis system. The first evidences for the anti-inflammatory mechanisms of PBMT were described by Lopes-Martins et al. (2006) and Marcos et al. (2011), showing that the laser inhibited cyclooxygenase–2 in inflamed tendons. A second hypothesis for the effects of lasers involves the ability of cytochrome c oxidase to absorb photons, leading to the photodissociation of inhibitory nitric oxide (NO), thus promoting an increase in mitochondrial membrane potential, oxygen consumption, and production of ATP (Chen et al. 2011; Huang et al. 2009; Hamblin 2017). These changes contribute to the induction of transcription factors, such as the nuclear factor kappa B (NF-κB), p53, activating transcription factor / cAMP responsive element binding protein (ATF/CREB), hypoxia-inducing factor (HIF)-1, which promote protein synthesis, increased proliferation, and migration of cells, thus modulating the levels of inflammatory mediators (Karu and Kolyakov 2005; Chung et al. 2012).
Recent studies have documented several beneficial effects of PBMT for neurological conditions, such as depression, traumatic brain injury, Parkinson’s disease, and Alzheimer’s disease (AD). Xuan et al. (2014) observed an improvement in spatial memory and learning of mice submitted to laser treatment four hours after traumatic brain injury. They also showed that PBMT stimulated neurogenesis in these mice. Cognitive performance improvement was also seen in animal models of AD accompanied by increased ATP levels, increased neuronal activation (c-fos), and decreased inflammatory markers (TNF-alpha and IL-1beta) (Taboada et al. 2011).
Despite the promising effects described above, most studies have evaluated PBMT only in neuropathological conditions (Oron et al. 2007; Taboada et al. 2011; Xuan et al. 2014). Thus, this study aimed to evaluate the neurobiological effect of PBMT on the healthy young and aged brain using a 100 mW power diode laser at 810 nm. Our hypothesis is that PBMT can improve the cognitive performance of rats in different stages of life (young and aged) and that the beneficial neurobiological effect may be related to the modulation of the neuroinflammatory profile in their cortex and hippocampus.
Materials and Methods
Animals
Sixty-four male Wistar rats, young (4 months old) and aged (20 months old), were used in this study. The animals were housed at a temperature of 21 ± 2 °C with a 12 h light/dark cycle (lights on from 7 am to 7 pm), and food and water were provided ad libitum throughout the experimental period. All procedures were approved by the ethics committee of the University of Mogi das Cruzes (UMC) (# 016/2017) and all effort was made to minimize animal suffering in accordance with the proposals of the International Ethical Guidelines for Biomedical Research (CIOMS 1985) (CIOMS, Council for International Organizations of Medical Sciences 1985).
Laser Therapy Protocol
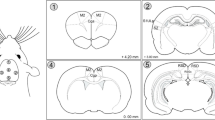
The rats were randomly distributed into four groups: young laser (YL; n = 15), young control (YC; n = 15), aged laser (AL; n = 18), and aged control (AC; n = 16). The animals from the laser groups (YL and AL) were manually immobilized and received the treatment with a laser diode of 810 nm wavelength and 100 mW power (DMC Equipment, São Carlos—Brazil) transcranially for 30 s (3 J of total energy/point) at each of the five irradiation points of application (point 1 = AP + 4.20 mm and ML 0.00 mm; point 2 = AP -3.00 mm and ML -6.60 mm; 3 = AP -3.00 mm and ML + 6.60 mm; point 4 = AP 0.00 mm and ML 0.00 mm; point 5 = AP -5.52 mm and ML 0.00 mm) (Fig. 1), totalizing 15 J of Energy, 150 s of irradiation, and fluency of 535,7 J/cm2 daily, between 12:00 and 1:00 pm. The animals in the control group were handled the same way except that the laser was off (placebo). Laser or placebo treatment was maintained throughout all the experiments until the animals were euthanized.
Behavioral Analyses
After 28 consecutive sessions of PBMT or placebo, the rats were submitted to behavioral tests performed sequentially in the following order: open field test, Barnes maze, elevated plus maze, and inhibitory avoidance. All behavioral procedures were conducted between 2:00 and 5:00 pm in a soundproof room. The behavioral analyses were performed independently by two investigators.
Thus, we had in the present study: 28 days of previous treatment with laser or placebo + 16 days of tests + five days of rest (four days between the open field and Barnes maze and one day between the elevated plus maze and inhibitory avoidance) + nine days of behavioral washout (in order to "wash out" the brain of the effects of behavioral tests) (Fig. 2).
Experimental design. Male Wistar rats from the young control (YC; n = 15), young laser (YL; n = 15), aged control (AC; n = 16), and aged laser (AL; n = 18) groups were exposed to a laser treatment or placebo over 58 consecutive days. From the 29th day of the experiment, the rats were submitted to four different behavior tests in the following sequence: exploratory activity and habituation in open field over three days, four days of rest, spatial learning and memory in the Barnes maze test for ten consecutive days (from the 36th to the 45th days of treatment), anxiety in elevated plus maze (46th day of treatment), one day of rest, aversive memory in inhibitory avoidance (48th and 49th days of treatment), and nine days of behavioral washout (from the 50th to the 58th day of treatment)
Open Field Test
The open field test was used to evaluate both exploratory activity and habituation. The apparatus consisted of a white circular arena (100 cm in diameter) made of acrylic polyvinyl. The floor of the arena was divided into 12 quadrants of equal area by lines. The animal was gently placed in the center of the arena to explore for 5 min. The rats were returned to their home cage immediately after the experiment finished. Crossings of quadrant lines were counted and used as measures of locomotion and exploration. To evaluate habituation, the experiment was repeated for three consecutive days. A decrease in the number of crossings throughout the three days of test indicates that the animal has been habituated, a type of non-associative memory (Vianna et al. 2000).
Barnes Maze
Learning and spatial memory was tested in a Barnes maze. The Barnes maze consists of a circular platform (1.22 m diameter) with 18 evenly spaced holes (9.5 cm diameter) at the circumference edge. Only one of the holes leads to an escape box (18 cm diameter) under the maze. Objects (pictures, frames, figures) were placed on the walls of the room to be used as clues. The animals were tested once a day for ten consecutive days and allowed to freely explore the maze for up to two minutes to locate the escape box. If the animal did not find the escape box, it was gently guided to the correct hole. The latency to escape was measured. Memory is reflected in a decrease of this latency throughout training.
Elevated Plus Maze
Anxiety was tested in elevated plus maze. The elevated plus maze consists of two opposite open arms (50 cm long, 10 cm wide) and two opposite closed arms (50 cm long, 10 cm wide, 40 cm high), elevated 50 cm above the floor. The rats were individually placed in the center of the maze with their heads facing the closed arm. During the behavioral test (5 min), the number of entries into the open and closed arms and the time spent on them were measured.
Inhibitory Avoidance
Aversive memory was tested in inhibitory avoidance. The inhibitory avoidance apparatus consists of an acrylic box (50 × 25 × 25 cm) with parallel stainless-steel bars (1 mm diameter) spaced 1 cm apart in the floor. A platform (7 × 25 cm) was placed against the left wall. For the inhibitory avoidance training, the animals were placed on the platform and allowed to step down onto the grid for up to 60 s. Immediately after stepping down on the grid with all four paws, animals received a 0.6 mA/s scrambled foot shock. The animals stayed in the apparatus for 60 s after the aversive stimulus was given and were immediately returned to their home cages. Ninety minutes (short-term memory) and 24 h (long-term memory) after the training session (aversive stimulus), the animals were again placed in the apparatus and the latency to step down was measured. If the animal did not step off the platform within 5 min, the experiment was terminated. In the long-term memory analysis, no aversive foot stimulus was delivered and the step-down latency was used to evaluate aversive memory.
Analysis of Chemokine and Cytokine Levels
Tissue Preparation
Twenty-four hours after the final laser session (59th day of experiment), the rats were euthanized by decapitation and their cerebral cortex and hippocampus were immediately collected and frozen. The tissue samples were homogenized in ice-cold RIPA lysis buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.5% sodium deoxycholate, 1% NP-40, 0.1% SDS) with freshly added protease (Cat# M222–1 ml; Lot# 1295C056; Amresco) and phosphatase (Cat# B15001-A and B; Lot# 510,011; Biotool) inhibitors. Homogenates were centrifuged at 10,000 × g for 10 min at 4 °C and supernatants were transferred to a new tube.
Cytokine and Chemokine Measurements
The levels of cytokines and chemokines in the brain samples were quantified using the Milliplex® MAP rat cytokine/chemokine magnetic bead panel assay (RECYTMAG-65 K, Merck Millipore) following the manufacturer’s specifications. This multiplex immunoassay allows the simultaneous quantification of 25 molecules: G-CSF, GM-CSF, eotaxin, IL-1alpha, leptin, MIP-1alpha, IL-4, IL-1beta, IL-2, IL-6, EGF, IL-13, IL-10, IL-12p70, IL-5, IL-17alpha, IL-18, MCP-1, IP-10, VEGF, fractalkine, LIX, MIP-2, TNF-alpha, and RANTES. The plates were read on a Luminex™ Magpix™ instrument and results were analyzed with the Milliplex Analyst 5.1 Software using a Logistic 5P Weighted regression formula to calculate sample concentrations from the standard curves.
Statistical Analysis
Statistical procedures were conducted using two-way ANOVA or two-way ANOVA with repeated measures. The Z-score was used to remove outlier values (± SEM): one outlier of leptin (AL), IL-5 (YC), IL-18 (AC), and RANTES (AL); two outliers of EGF (YC and YL) and IP-10 (YC and AC) in the cortex; and one outlier of GM-CSF (YL), IL-2 (YL), EGF (YC), IL-12p70 (YC), IL-5 (AL), and fractalkine (YC) in the hippocampus. All analyses were performed using the Statistical Package for the Social Science (SPSS Inc, IBM, version 221.0, Chicago, IL, USA). A statistical difference was considered when the P-value was lower than 0.05. All plots were acquired using the GraphPad Prism (6.0).
Results
Exploratory Activity in Open Field
Exploratory activity was measured in an open field apparatus on the 29th day of the experiment. Two-way ANOVA showed a significant difference in age [central locomotion (F(1,60) = 7.309; p = 0.009), peripheral locomotion (F(1,60) = 14.139; p < 0.0001), and total locomotion (F(1,60) = 15.543; p < 0.0001)], but no significant effect in group [central locomotion (F(1,60) = 0.201; p = 0.655), peripheral locomotion (F(1,60) = 0.007; p = 0.934), and total locomotion (F(1,60) < 0.0001; p = 1.000)] and interaction (age*group) [central locomotion (F(1,60) = 1.048; p = 0.310), peripheral locomotion (F(1,60) = 0.637; p = 0.428), and total locomotion (F(1,60) = 0.835; p = 0.365)] (Fig. 3). These data suggest that the laser exerts no effect on the locomotion and exploration of young and aged rats in the open field test.
Open field quadrants (left) and exploratory activity from young control (YC; n = 15), young laser (YL; n = 15), aged control (AC; n = 16), and aged laser (AL; n = 18) groups. Exploratory activity is expressed as number of quadrants crossed (locomotion units) for both the four central quadrants (center) and the eight peripheral quadrants (periphery). Total locomotion corresponds to the sum of central and peripheral locomotion. No significant effect was observed (two-way ANOVA)
Habituation in Open Field
Habituation was measured in the open field apparatus over three days (from the 29th to the 31st day of treatment). Statistical analysis of central, peripheral, and total locomotion data was conducted independently by ANOVA with repeated measures. In the central locomotion variable, an effect of day was observed (F(2,120) = 4.641; p = 0.011), but not group (F(3,60) = 1.379; p = 0.258) and interaction (F(6,120) = 1.534; p = 0.173). In the peripheral locomotion variable, an effect of day (F(2,120) = 4.402; p = 0.014) and group (F(3,60) = 5.913; p = 0.001) was observed, but not interaction (F(6,120) = 0.560; p = 0.761). In the total locomotion variable, an effect of day (F(2,120) = 5.565; p = 0.005) and group (F(3,60) = 5.526; p = 0.002) was observed, but not interaction (F(6,120) = 0.615; p = 0.718) (Fig. 4). These data suggest that the laser does not alter the habituation (non-associative memory task) of young and aged rats.
Habituation memory from young control (YC; n = 15), young laser (YL; n = 15), aged control (AC; n = 16), and aged laser (AL; n = 18) groups over three consecutive days. No significant difference in central, peripheral, or total locomotion was found among groups (two-way ANOVA with repeated measures)
Spatial Learning and Memory in the Barnes Maze
Spatial learning and memory were assessed in the Barnes maze for ten consecutive days (from the 36th to the 45th day of treatment). Two-way ANOVA with repeated measures showed an effect of day (F(9,540) = 72.301; p < 0.0001), group (F(3,60) = 2.972; p = 0.039), and interaction (group*day) (F(27,540) = 3.473; p < 0.0001). When Bonferroni post hoc analysis was performed, it was noted that young groups [YC (p = 0.034), YL (p < 0.0001)] and AL group (p = 0.003) learned to find the true hole earlier (on the 6th day of testing) than the AC group (learning of the task in this group occurred only on the 7th test day; p = 0.003) (Fig. 5).
A schematic representation of the Barnes maze (left) and latency to find the hole over the ten consecutive days (120 s/day) of a test in the Barnes labyrinth in animals of all groups: young control (YC; n = 15), young laser (YL; n = 15), aged control (AC; n = 16), and aged laser (AL; n = 18). A statistical difference was observed on the sixth test day in relation to the first day in the animals of the YC (▯), YL (▯) and AL (◆) groups; in the AC group this difference was observed on the seventh test day (◆). In addition, on the eighth day of the test, a significant decrease in latency was noted to find the true hole between the YL and YC groups (#). This same effect was found in aged rats because there was a significant reduction in the mnemonic performance of the animals of the AL group compared to the animals of the AC group on the ninth and tenth days (##) (p < 0,05; two-way ANOVA with repeated measures)
In addition, on the 8th day of the test, a better performance in finding the true hole was noted in the YL group in comparison to the YC group (p = 0.002). This was also found in the aged animals because a lower latency was observed in the AL group when compared to the AC group on the 9th (p = 0.001) and 10th (p = 0.045) days of the test. These data show that animals in the PBMT group showed better performance in finding the true hole over the test days than the control group. In addition, the PBMT group was able to rejuvenate the spatial mnemonic damage of the aged rats, resulting in a performance close to that of the rats in the YC group (Fig. 5).
Anxiety in Elevated Plus Maze
Anxiety was assessed using the elevated plus maze where rats can freely explore closed or open arms on a high platform (46th day of treatment). An increase in time spent in the closed arms is related to anxiety. The statistical analyses were performed by two-way ANOVA. No significant effect was observed in the variables analyzed: time on closed arms [age (F(1,60) = 2.808; p = 0.099), group (F(1,60) = 2.013; p = 0.161), and interaction (F(1,60) = 1.350; p = 0.250)]; time on open arms [age (F(1,60) = 2.021; p = 0.160), group (F(1,60) = 0.023; p = 0.881), and interaction (F(1,60) = 0.268; p = 0.607)]; entries on closed arms [age (F(1,60) = 0.477; p = 0.493), group (F(1,60) = 3.267; p = 0.076), and interaction (F(1,60) = 3.460; p = 0.068)]; and entries on open arms [age (F(1,60) = 1.749; p = 0.191), group (F(1,60) = 0.045; p = 0.833), and interaction (F(1,60) < 0.0001; p = 0.995)] (Fig. 6). These data suggest that PBMT exerts no anxiolytic effect on the elevated plus maze test in young and aged rats.
Aversive Memory in Inhibitory Avoidance
Aversive memory was measured in the inhibitory avoidance (48th and 49th days of treatment). The step-down latency was used to evaluate short- (90 min after the training session) and long-term (24 h after the training session) aversive memory. Two-way ANOVA showed no significant difference in age (F(1,57) = 0.737; p = 0.394), group (F(1,57) = 0.463; p = 0.499), and interaction (F(1,57) = 0.273; p = 0.604) when the short-term memory was evaluated (Fig. 7a). When long-term memory was evaluated, a significant difference was found between groups (F(1,57) = 9.856; p = 0.003), but not in age (F(1,57) = 2.213; p = 0.142) or interaction (F(1,57) = 0.448; p = 0.506) (Fig. 7b). These data show that the laser has no effect on the short- and long-term aversive memory.
Cortical and Hippocampal Levels of Cytokines and Chemokines
The results of the two-way ANOVA are presented in Supplementary Tables S1, S2, S3, and S4. No significant effect was observed in the cortical levels of.
G-CSF (F(1,21) = 0.504; p = 0.486), eotaxin (F(1,21) = 3.088; p = 0.093), IL-1alpha (F(1,21) = 2.323; p = 0.127), leptin (F(1,20) = 1.246; p = 0.278), MIP-1alpha (F(1,21) = 0.399; p = 0.535), IL-4 (F(1,21) = 1.202; p = 0.285), IL-1beta (F(1,21) = 1.267; p = 0.273), IL-2 (F(1,21) = 0.031; p = 0.862), EGF (F(1,19) = 1.963; p = 0.177), IL-13 (F(1,21) = 3.849; p = 0.063), IL-17alpha (F(1,21) = 0.015; p = 0.902), IL-18 (F(1,20) = 0.180; p = 0.676), IP-10 (F(1,19) = 1.402; p = 0.251), VEGF (F(1,21) = 0.052; p = 0.822), fractalkine (F(1,21) = 0.884; p = 0.358), MIP-2 (F(1,21) = 2.308; p = 0.144), and RANTES (F(1,20) = 0.965; p = 0.338). In the other cytokines/chemokines, we noted significant effect (p < 0.05). When Bonferroni post hoc analysis was performed, we noted that laser treatment reduced the cortical levels of GM-CSF (p < 0.0001), IL-10 (p = 0.003), MCP-1 (p = 0.012), LIX (p = 0.006), and TNF (p < 0.0001) in young rats. Also, we noted a decrease in the cortical levels of GM-CSF (p < 0.0001), IL-6 (p = 0.005), MCP-1 (p = 0.028), LIX (p < 0.0001), and TNF (p < 0.0001) in the animals of the AC group compared to the animals of the YC group. In addition, the laser increased IL-6 (p = 0.046), IL-10 (p = 0.008), and TNF-alpha (p = 0.027) levels and reduced IL-5 cortical levels in aged rats (p = 0.005) (Fig. 8). Despite finding a significant difference in the two-way ANOVA in the cortical levels of IL-12p70, no effect was observed in the Bonferroni post hoc analysis.
Cortical levels of GM-CSF, IL-6, IL-10, IL-5, MCP-1, LIX, and TNFα in rats from young control (YC; n = 7), young laser (YL; n = 7), aged control (AC; n = 5), and aged laser (AL; n = 6) groups. A significant decrease in GM-CSF, IL-10, MCP-1, LIX, and TNF levels were found in the YL group when compared to the YC group (#). A significant decrease in the levels of GM-CSF, IL-6, MCP-1, LIX, and TNF were noted in the AC group, compared to the YC group (#). In addition, the laser increased IL-6, IL-10, and TNFα levels and reduced IL-5 levels in aged rats (##) (p < 0,05; two-way ANOVA)
In the hippocampus, no effect was noted in the G-CSF (F(1,20) < 0.0001; p = 0.988), GM-CSF (F(1,19) = 3.447; p = 0.079), eotaxin (F(1,20) = 0.002; p = 0.964), IL-1alpha (F(1,20) = 3.194; p = 0.089), leptin (F(1,20) = 0.018; p = 0.896), MIP-1alpha (F(1,20) = 2.125; p = 0.160), IL-4 (F(1,20) = 0.191; p = 0.667), IL-1beta (F(1,20) = 0.203; p = 0.657), IL-2 (F(1,19) = 1.074; p = 0.313), IL-6 (F(1,20) = 0.202; p = 0.658), EGF (F(1,19) = 2.319; p = 0.144), IL-13 (F(1,20) = 0.080; p = 0.780), IL-10 (F(1,20) = 1.003; p = 0.329), IL-12p70 (F(1,19) = 0.270; p = 0.610), IL-5 (F(1,19) = 0.069; p = 0.795), IL-17alpha (F(1,20) = 0.160; p = 0.694), IL-18 (F(1,20) = 1.472; p = 0.239), MCP-1 (F(1,20) = 0.472; p = 0.500), VEGF (F(1,20) = 3.878; p = 0.063), LIX (F(1,20) = 0.141; p = 0.711), MIP-2 (F(1,20) = 1.639; p = 0.215), TNF-alpha (F(1,20) = 0.430; p = 0.520), and RANTES (F(1,20) = 4.189; p = 0.054) levels. However, was observed a significant effect in the IP-10 (F(1,20) = 4.892; p = 0.039) and fractalkine (F(1,19) = 5.819; p = 0.026). When Bonferroni post hoc analysis was performed, an increase in the IP-10 levels was observed in control aged rats when compared to control young rats (p = 0.025). Interestingly, the laser was able to reduce the exacerbated levels of IP-10 (p = 0.038) back to levels seen in young animals. In addition, PBMT reduced the level of fractalkine (p = 0.033) from aged rats (Fig. 9). Taken together, these data show that PBMT changes the levels of neuroinflammatory markers in young and aged rats (Table 1).
Hippocampal levels of IP-10 and Fractalkine in rats from young control (YC; n = 7), young laser (YL; n = 7), aged control (AC; n = 5), and aged laser (AL; n = 5) groups. A significant increase in IP-10 levels was found in the AC group when compared to the YC group (#). A significant decrease in IP-10 and Fractalkine levels were noted in the AL group compared to the AC group (##) (p < 0,05; two-way ANOVA)
Discussion
The purpose of our study was to investigate the cognitive performance and the levels of pro- and anti-inflammatory cytokines and chemokines in the brain of young (4 months) and aged (20 months) rats exposed to a chronic treatment with a diode laser of 810 nm wavelength and 100 mW power. Our results indicate that PBMT was able to improve learning and spatial memory and modulate the neuroinflammatory profile of young and aged rats.
Photobiomodulation and Behavioral Tests
In the present study, habituation memory in the open field, anxiety in the elevated plus maze test, and short- and long-term aversive memory in the inhibitory avoidance were not significantly altered by PBMT. Our study is the first to evaluate the habituation memory, anxiety, and aversive memory of aged rats exposed to laser treatment. However, these results are intriguing, since previous studies showed beneficial effects of laser treatment in the cognition of different animal models (Xu et al. 2017; Salehpour et al. 2017, 2019). For example, Xu and colleagues (2017) showed that an animal model of anxiety (Ahi1 knockout mice) exhibited fewer anxiety symptoms in the forced swimming test and tail suspension 7, 14, and 21 days after laser treatment. Based on the data described in the literature, we expected that the laser therapy protocol used in our study could improve behavioral performance of young and aged rats on open field, elevated plus maze, and step-down inhibitory avoidance apparatus. We found no effect of PBMT on these tests. We only noted PBMT-induced benefits in the Barnes maze test. Young and aged rats submitted to transcranial laser showed better performance in finding the true hole over the days of the test than control rats. This laser benefit on rat’s spatial memory in the Barnes maze was also observed in a previous study conducted in an animal model of aging (D-galactose-induced aging (Salehpour et al. 2017) and of transient global brain ischemia (Salehpour et al. 2019).
Photobiomodulation and Inflammatory Response in Young Rats
The laser treatment altered the cortical and hippocampal expression of inflammatory markers. The laser reduced the cortical levels of GM-CSF, MCP1, LIX, and TNF-alpha in young rats. These results are optimistic, since GM-CSF is related to several infectious and inflammatory diseases (Shim et al. 2012). For example, Shang et al. (2016) noted that high production of GM-CSF in the brain parenchyma of AD patients promotes monocyte transmigration across the blood–brain barrier. MCP-1 induces expression and secretion of RANTES chemokine in T cells and MIP-3α in brain ischemia model animals, increasing inflammatory response (Che et al. 2001; Chen et al. 2003; Terao et al 2009). The expression of LIX during brain ischemia, close to its receptor, measures the activation of JNK and p38 signal pathways which are linked to inflammation and cell death (Jeyaseelan et al. 2005; Shin et al. 2014). TNFα is a pro-inflammatory cytokine involved in innate immune response (Clark 2007) that encodes inflammatory enzymes, such as inducible nitric oxide (iNOS) and cyclooxygenase-2 (COX-2), related to various neurodegenerative diseases, such as AD (Allan and Rothwell 2003; Heneka 2006).
Photobiomodulation and Inflammatory Response in Aged Rats
Our data show that laser treatment alters the inflammatory response of aged rats, corroborating with studies that show these effects on the retina (Kokkinopoulos et al. 2013; Sivapathasuntharam et al. 2017) and brain (El Massri et al 2018) of aged animals. In our study, the laser increased the cortical expression of IL-6, TNF-alpha, and IL-10 in aged rats. IL-6 has pro and anti-inflammatory properties (Scheller et al 2011; Ataie-Kachoie et al. 2014; Yao et al. 2014) For example, this cytokine is involved in the activation of the immune system, but also in the regulation of metabolism and in many neural functions (Scheller et al. 2011). These effects depend on the activation mechanisms and length of exposure to the cytokine (Ataie-Kachoie et al. 2014). One hypothesis for this may be the anti-inflammatory effect of PBMT, since the reduced IL-6 cortical levels in aged rats were restored by laser treatment. TNF-alpha is up-regulated in many degenerative diseases. However, the TNF-alpha can exert a neuroprotection mediated by TNFR2 activation against glutamate-induced excitotoxicity (Marchetti et al. 2004). This cytokine induces persistent NF-kB activation involving PI3K and Akt, which is strongly enhanced by N-methyl-D-aspartate receptor activation, in turn is essential for neuronal survival and synaptic plasticity (Marchetti et al. 2004; Mattson 2005). Furthermore, these data are consistent with a study conducted by our research group. We noted that laser treatment reduced the cortical metabolic pathway of glutamate in aged rats (Cardoso et al. 2021). IL-10 is an anti-inflammatory cytokine that inhibits the activation of pro-inflammatory proteins (Ye and Johnson 2001). Studies show that IL-10 blockage triggers neuronal damage and behavioral impairment (Grilli et al. 2000; Krzyszton et al. 2008). These data suggest that during aging, reduced levels of IL-10 may result in vulnerability and neuronal dysfunction.
Supporting the positive effect of PBMT on the aged brain, the laser therapy reduced the cortical expression of IL-5 in aged rats. IL-5 is a pro-inflammatory cytokine that induces proliferation of microglia and increases IL-9 levels under excitotoxic conditions (Liva and Vellis 2001). In this sense, the aged brain is characterized by microglia reactivity and high levels of pro-inflammatory cytokines (Jurgens and Johnson 2012). Moreover, these findings corroborate studies of gene expression profiles of human brains and aged animals. It is observed that the expression of genes involved in oxidative stress, inflammation, and glial activation increases during the aging process, while genes linked to synaptic function and growth factors decrease throughout life (Bishop et al. 2010; Blalock et al. 2003; Godbout et al. 2005; Lee et al. 2000).
In the hippocampus, we found an increase in IP-10 expression in rats from the AC group compared to rats from the YC group. Interestingly, the laser therapy reduced exacerbated levels of this chemokine in aged rats. In addition, PBMT also reduced fractalkine levels in aged rats. These results are interesting since studies have shown that these chemokines are elevated in the brains of animals with AD (Hanzel et al. 2014; Scholtzova et al. 2014). Moreover, it is noted that Aβ plaques can stimulate the production of IP-10 and fractalkine, along with memory loss (Lai et al. 2013; Wu et al. 2013). It is observed that increased expression of chemokines and cytokines induces neuritic dystrophy, triggering increased expression and phosphorylation of neurofilaments and tau protein (Sheng et al. 2000). In addition, chemokines can interact with each other. It is known that the interaction between SDF-1α and CXCR4 in astroglioma cells induces the expression of MCP-1, IL-8, and IP-10 through the ERK signaling pathway, which is linked to inflammation and angiogenesis (Oh et al. 2001).
Impact of Photobiomodulation on Young and Old Brain
Our data show that the greatest effects of PBMT on behavioral performance and neuroinflammatory response were in aged rats, corroborating with the findings of Shinhmar and collaborators (2020). They noted that laser treatment improved the rod and cone function in elderly people, but not in younger individuals, possibly, because age-related mitochondrial decline has not yet impacted young individuals (Shinhmar et al. 2020).
Limitations
Although our data are promising, it is worth noting that the study has limitations. We cannot affirm that our behavioral and inflammatory results are closely related. In other words, we cannot assume that the behavioral performance of the animals is linked to changes in brain inflammatory levels since our analyses occurred on different days. The behavioral analyses were performed from the 29th to the 49th days of treatment, while biochemical analyses were performed on the 58th day of treatment.
Conclusion
Despite that, we consider transcranial photobiomodulation to be a non-pharmacological therapeutic tool with potential for age-related brain disorders, mainly for improving memory and restoring inflammatory levels.
References
Albertini R, Aimbire FSC, Correa FI, Ribeiro W, Cogo JC, Antunes E, Teixeira SA, De Nucci G, Castro-Faria-Neto HC, Zângaro RA, Lopes-Martins RAB (2004) Effects of different protocol doses of low power gallium–aluminum–arsenate (Ga–Al–As) laser radiation (650 nm) on carrageenan induced rat paw ooedema. J Photochem Photobiol, B 74(2–3):101–107
Allan SM, Rothwell NJ (2003) Inflammation in central nervous system injury. Philos Trans R Soc Lond B Biol 358(1438):1669–1677
Ataie-Kachoie P, Pourgholami MH, Richardson DR, Morris DL (2014) Gene of the month: Interleukin 6 (IL-6). J Clin Pathol 67(11):932–937
Bishop NA, Lu T, Yankner BA (2010) Neural mechanisms of ageing and cognitive decline. Nature 464(7288):529
Bjordal JM, Johnson MI, Iversen V, Aimbire F, Lopes-Martins RA (2006a) Low-level laser therapy in acute pain: a systematic review of possible mechanisms of action and clinical effects in randomized placebo-controlled trial. Photomedicine and Laser theapy 24(2):158–168
Bjordal JM, Lopes-Martins RA, Iversen VV (2006b) A randomised, placebo controlled trial of low level laser therapy for activated Achilles tendinitis with microdialysis measurement of peritendinous prostaglandin E2 concentrations. Br J Sports Med 40(1):76–80
Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW (2003) Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci 23(9):3807–3819
Cardoso FDS, França EF, Serra FT, Victorino AB, de Almeida AA, Fernandes J, Gomes da Silva S (2017) Aerobic exercise reduces hippocampal ERK and p38 activation and improves memory of middle-aged rats. Hippocampus 27(8):899–905
Cardoso FDS, Santos JCC, Gonzalez-Lima F, Araújo BHS, Lopes-Martins RÁB, Gomes da Silva S (2021) Effects of Chronic Photobiomodulation with Transcranial Near-Infrared Laser on Brain Metabolomics of Young and Aged Rats. Mol Neurobiol. https://doi.org/10.1007/s12035-020-02247-z
Castle SC (2000) Clinical relevance of age-related immune dysfunction. Clin Infect Dis 31(2):578–585
Che X, Ye W, Panga L, Wu DC, Yang GY (2001) Monocyte chemoattractant protein-1 expressed in neurons and astrocytes during focal ischemia in mice. Brain Res 902(2):171–177
Chen AC, Arany PR, Huang YY, Tomkinson EM, Sharma SK, Kharkwal GB, Saleem T, Mooney D, Yull FE, Blackwell TS, Hamblin MR (2011) Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS ONE. https://doi.org/10.1371/journal.pone.0022453
Chen Y, Hallenbeck JM, Ruetzler C, Bol D, Thomas K, Berman NE, Vogel SN (2003) Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J Cereb Blood Flow Metab 23(6):748–755
Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR (2012) The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 40(2):516–533
Clark IA (2007) How TNF was recognized as a key mechanism of disease. Cytokine Growth Factor Rev 18(3–4):335–343
Council for International Organizations of Medical Sciences (1985) International guiding principles for biomedical research involving animals. Altern Lab Anim 12:ii
El Massri N, Weinrich TW, Kam JH, Jeffery G, Mitrofanis J (2018) Photobiomodulation reduces gliosis in the basal ganglia of aged mice. Neurobiol Aging 66:131–137
Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW (2005) Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J 19(10):1329–1331
Godbout JP, Johnson RW (2009) Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Immunol Allergy Clin North Am 29(2):321–337
Grilli M, Barbieri I, Basudev H, Brusa R, Casati C, Lozza G, Ongini E (2000) Interleukin-10 modulates neuronal threshold of vulnerability to ischaemic damage. Eur J Neurosci 12(7):2265–2272
Hamblin MR (2017) Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS biophysics 4(3):337
Hanzel CE, Pichet-Binette A, Pimentel LS, Iulita MF, Allard S, Ducatenzeiler A, Cuello AC (2014) Neuronal driven pre-plaque inflammation in a transgenic rat model of Alzheimer’s disease. Neurobiol Aging 35(10):2249–2262
Heneka MT (2006) Inflammation in Alzheimer’s disease. Clin Neurosci Res 6(5):247–260
Huang YY, Chen ACH, Carroll JD, Hamblin MR (2009) Biphasic dose response in low level light therapy. Dose-Response. https://doi.org/10.2203/dose-response.09-027.Hamblin
Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, Worthen GS (2005) Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. Am J Respir Cell Mol Biol 32(6):531–539
Jurgens HA, Johnson RW (2012) Dysregulated neuronal–microglial cross-talk during aging, stress and inflammation. Exp Neurol 233(1):40–48
Karu TI, Kolyakov SF (2005) Exact action spectra for cellular responses relevant to phototherapy. Photomedicine and Laser Therapy 23(4):355–361
Kokkinopoulos I, Colman A, Hogg C, Heckenlively J, Jeffery G (2013) Age-related retinal inflammation is reduced by 670 nm light via increased mitochondrial membrane potential. Neurobiol Aging 34(2):602–609
Krzyszton CP, Sparkman NL, Grant RW, Buchanan JB, Broussard SR, Woods J, Johnson RW (2008) Exacerbated fatigue and motor deficits in interleukin-10-deficient mice after peripheral immune stimulation. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 295(4):R1109–R1114
Lai W, Wu J, Zou X, Xie J, Zhang L, Zhao X, Ji J (2013) Secretome Analyses of Aβ1–42 Stimulated Hippocampal Astrocytes Reveal that CXCL10 is Involved in Astrocyte Migration. J Proteome Res 12(2):832–843
Lee CK, Weindruch R, Prolla TA (2000) Gene-expression profile of the ageing brain in mice. Nat Genet 25(3):294
Liva SM, de Vellis J (2001) IL-5 induces proliferation and activation of microglia via an unknown receptor. Neurochem Res 26(6):629–637
Lopes-Martins RAB, Albertini R, Lopes-Martins PSL, Carvalho FAS, Neto HCCF, Iversen VV, Bjordal JM (2006) Steroid receptor antagonist mifepristone inhibits the anti-inflammatory effects of photoradiation. Photomedicine and Laser Therapy 24(2):197–201
Lopes-Martins RAB, Albertini R, Lopes PSL, Bjordal JM, Neto HCCF (2005) Spontaneous effects of low-level laser therapy (650 nm) in acute inflammatory mouse pleurisy induced by carrageenan. Photomedicine and Laser Therapy 23(4):377–381
Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL (2004) Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation: essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-κB pathway. J Biol Chem 279(31):32869–32881
Marcos RL, Leal Junior ECP, de Moura Messias F, Catelli de Carvalho MH, Pallotta RC, Frigo L, dos Santos RA, Ramos L, Teixeira S, Bjordal JM, Lopes-Martins RAB (2011) Infrared (810 nm) low-level laser therapy in rat Achilles tendinitis: a consistent alternative to drugs. Photochem Photobiol 87(6):1447–1452
Mattson MP (2005) NF-κB in the survival and plasticity of neurons. Neurochem Res 30(6):883–893
Mattson MP, Magnus T (2006) Ageing and neuronal vulnerability. Nat Rev Neurosci 7(4):278
Oh JW, Drabik K, Kutsch O, Choi C, Tousson A, Benveniste EN (2001) CXC chemokine receptor 4 expression and function in human astroglioma cells. J Immunol 166(4):2695–2704
Olazaran J, Muñiz R, Reisberg B, Peña-Casanova J, Del Ser T, Cruz-Jentoft AJ, Galiano M (2004) Benefits of cognitive-motor intervention in MCI and mild to moderate Alzheimer disease. Neurology 63(12):2348–2353
Oron A, Oron U, Streeter J, Taboada LD, Alexandrovich A, Trembovler V, Shohami E (2007) Low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits. J Neurotrauma 24(4):651–656
Rozzini L, Costardi D, Chilovi BV, Franzoni S, Trabucchi M, Padovani A (2007) Efficacy of cognitive rehabilitation in patients with mild cognitive impairment treated with cholinesterase inhibitors. Int J Geriat Psychiatry 22(4):356–360
Salehpour F, Ahmadian N, Rasta SH, Farhoudi M, Karimi P, Sadigh-Eteghad S (2017) Transcranial low-level laser therapy improves brain mitochondrial function and cognitive impairment in D-galactose–induced aging mice. Neurobiol Aging 58:140–150
Salehpour F, Farajdokht F, Mahmoudi J, Erfani M, Farhoudi M, Karimi P, Rasta SH, Sadigh-Eteghad S, Hamblin MR, Gjedde A (2019) Photobiomodulation and Coenzyme Q10 Treatments Attenuate Cognitive Impairment Associated with Model of Transient Global Brain Ischemia in Artificially Aged Mice. Front Cell Neurosci. https://doi.org/10.3389/fncel.2019.00074
Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S (2011) The pro-and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1813(5):878–888
Scholtzova H, Chianchiano P, Pan J, Sun Y, Goñi F, Mehta PD, Wisniewski T (2014) Amyloid β and Tau Alzheimer’s disease related pathology is reduced by Toll-like receptor 9 stimulation. Acta neuropathologica communications 2(1):101
Shang DS, Yang YM, Zhang H, Tian L, Jiang JS, Dong YB, Chen YH (2016) Intracerebral GM-CSF contributes to transendothelial monocyte migration in APP/PS1 Alzheimer’s disease mice. J Cereb Blood Flow Metab 36(11):1978–1991
Shinhmar H, Grewal M, Sivaprasad S, Hogg C, Chong V, Neveu M, Jeffery G (2020) Optically improved mitochondrial function redeems aged human visual decline. J Gerontol A-Biol 75(9):e49–e52
Sivapathasuntharam C, Sivaprasad S, Hogg C, Jeffery G (2017) Aging retinal function is improved by near infrared light (670 nm) that is associated with corrected mitochondrial decline. Neurobiol Aging 52:66–70
Sheng JG, Zhu SG, Jones RA, Griffin WST, Mrak RE (2000) Interleukin-1 promotes expression and phosphorylation of neurofilament and tau proteins in vivo. Exp Neurol 163(2):388–391
Shim DS, Schilter HC, Knott ML, Almeida RA, Short RP, Mackay CR, Sewell WA (2012) Protection against Nippostrongylusbrasiliensis infection in mice is independent of GM-CSF. Immunol Cell Biol 90(5):553–558
Shin JH, Park YM, Kim DH, Moon GJ, Bang OY, Ohn T, Kim HH (2014) Ischemic brain extract increases SDF-1 expression in astrocytes through the CXCR2/miR-223/miR-27b pathway. Biochimica et Biophysica Acta (BBA)-Gene Regulatory Mechanisms 1839(9):826–836
Taboada L, Yu J, El-Amouri S, Gattoni-Celli S, Richieri S, McCarthy T, Streeter J, Kindy MS (2011) Transcranial laser therapy attenuates amyloid-β peptide neuropathology in amyloid-β protein precursor transgenic mice. J Alzheimers Dis 23(3):521–535
Terao Y, Ohta H, Oda A, Nakagaito Y, Kiyota Y, Shintani Y (2009) Macrophage inflammatory protein-3alpha plays a key role in the inflammatory cascade in rat focal cerebral ischemia. Neurosci Res 64(1):75–82
Vianna MR, Alonso M, Viola H, Quevedo J, De Paris F, Furman M, Izquierdo I (2000) Role of hippocampal signaling pathways in long-term memory formation of a nonassociative learning task in the rat. Learn Mem 7(5):333–340
Wu J, Bie B, Yang H, Xu JJ, Brown DL, Naguib M (2013) Suppression of central chemokine fractalkine receptor signaling alleviates amyloid-induced memory deficiency. Neurobiol Aging 34(12):2843–2852
Xu Z, Guo X, Yang Y, Tucker D, Lu Y, Xin N, Zhang G, Yang L, Li J, Du X, Zhang Q, Xu X (2017) Low-level laser irradiation improves depression-like behaviors in mice. Mol Neurobiol 54(6):4551–4559
Xuan W, Vatansever F, Huang L, Hamblin MR (2014) Transcranial low-level laser therapy enhances learning, memory, and neuroprogenitor cells after traumatic brain injury in mice. J Biomed Opt 19(10):108003
Yao X, Huang J, Zhong H, Shen N, Faggioni R, Fung M, Yao Y (2014) Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther 141(2):125–139
Ye SM, Johnson RW (2001) An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. NeuroImmunoModulation 9(4):183–192
Acknowledgements
This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Centros de Pesquisa, Inovação e Difusão (CEPID; #2013/08028-1), and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; #2017/16443-0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest. All the authors read and approved the final manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cardoso, F.S., de Souza Oliveira Tavares, C., Araujo, B.H.S. et al. Improved Spatial Memory And Neuroinflammatory Profile Changes in Aged Rats Submitted to Photobiomodulation Therapy. Cell Mol Neurobiol 42, 1875–1886 (2022). https://doi.org/10.1007/s10571-021-01069-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-021-01069-4