Abstract
Glioma is a highly malignant type of intracranial tumor with a poor prognosis resulting from traditional chemo-resistance with temozolomide (TMZ). Luteolin has been detected to exert limited anti-tumor effects on gliomas, while valproic acid (VPA) is a common chemotherapy sensitizer in the treatment of tumors. In this study, three glioma cell lines including U251, LN229 and SNB19 were selected for evaluation of combined anti-tumor effects of VPA and luteolin via Cell Counting Kit-8 (CCK-8) assay, colony formation assay, wound-healing assay, flow cytometry and western blot assay. The results disclosed that VPA sensitized glioma cells to luteolin by repressing cell viability, colony formation and migration. Mechanically, VPA boosted cellular apoptosis and cell-cycle arrest by increased level of cleaved caspase-3/caspase-3, cleaved PARP/PARP and Bax/Bcl-2. In addition, VPA also facilitated cellular autophagy via the decline of p62, p-Akt/Akt and the accumulation of LC3-II. These findings suggested that VPA enhanced the anticancer effects of luteolin by strengthening apoptosis and autophagy via Akt signaling, which could be adopted as a novel therapy for glioma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioma, considered as a common and highly malignant type of intracranial tumor, accounts for 60% of central nervous system (CNS) tumors (Geng et al. 2019; Xiong et al. 2019). Despite the progress in elucidating the biological mechanisms and improving the treatment of glioma, the clinical prognosis is still poor with a median survival time of 14.6 months (Cao et al. 2019). Luteolin is a common flavonoid extracted from salvia tomentosa mill and many other plants and vegetables (Anson et al. 2018), which has been more and more promising for its anti-tumor effects in various solid tumors including bladder cancer (Iida et al. 2020), prostate cancer (Naiki-Ito et al. 2019), breast cancer (Gao et al. 2019), colorectal cancer (Yao et al. 2019), etc. Interestingly, luteolin was also observed to exert inhibitory effects on glioma including suppressing cell viability, migration, invasion and angiogenesis, while inducing cell-cycle arrest and apoptosis (You et al. 2019; El Gueder et al. 2018; Anson et al. 2018). However, the hydrophobic and poor biocompatibility of luteolin leads to its low bioavailability (Zheng et al. 2017), predicting the biggest obstacle on further clinical application. Though a study by Wu et.al illustrated that luteolin with nanoparticle modification significantly enhanced its anti-glioma effect (Wu et al. 2019), chemosensitization with higher penetration is still our top priority. Therefore, it is an urgent need to apply novel drugs with curative effects and no side effects to sensitize glioma cells to luteolin treatment.
Valproic acid (VPA), one of common histone deacetylases (HDACs), is a clinically anti-convulsant and mood-stabilizing drug (Eckert et al. 2017). Resulting from its anti-tumor or sensibilization activities in multiple tumors (Wu et al. 2016; Riva et al. 2018; Tseng et al. 2017), more and more attention has been attracted to subsequent clinical trials. For instance, VPA significantly inhibited cell viability and invasion through upregulation of n-myc downstream regulated gene-1 (NDRG1) in prostate cancer (Lee and Kim 2015). In thyroid cancer, VPA exerted anti-tumor effects by inducing apoptosis and autophagy by the inhibition of RET signaling (Xu et al. 2015). As in glioma, VPA promoted cellular apoptosis and inhibited glycogen synthase kinase-3β (GSK-3β) through ERK/Akt signaling (Zhang et al. 2016). However, apart from direct inhibition of VPA on human cancers, the capacity of VPA to sensitize chemotherapy drugs was more widely acknowledged. Chen et.al revealed that VPA enhanced cisplatin sensitivity to non-small cell lung cancer cells (NSCLCs) via HDAC2 mediated downregulation of ATP-binding cassette transporter A1 (ABCA1) (Chen et al. 2017). Interestingly, combination chemotherapy of VPA and gemcitabine regulated signal transducer and activator of transcription 3 (STAT3)/Bmi1 pathway to inhibit the migration and invasion of pancreatic cancer cells (Li et al. 2019). Moreover, VPA sensitized glioma cells to sulfasalazine in promoting cell death through imbalance of the intracellular oxidative response (Garcia et al. 2018). Therefore, the combination chemotherapy of VPA and luteolin could be a potential novel therapy for glioma.
In this study, we explored that VPA efficiently sensitized glioma cells to luteolin in inhibiting cell viability, cellular colony formation and migration and inducing cell-cycle arrest in glioma. Moreover, the sensitization of VPA could be attributed to the induction of apoptosis and autophagy via the suppression of the Akt signaling. All the above results indicated that VPA sensitized glioma cells to luteolin through induction of apoptosis and autophagy via Akt signaling, providing a novel and reliable therapy for glioma.
Materials and Methods
Cell Culture and Materials
The glioma cell lines U251, LN229 and SNB19 and human HEB cells were purchased from the Chinese Academy of Science’s Cell Bank (Shanghai, China). The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and antibiotics including 100 U/mL penicillin and 100 mg/L streptomycin in a 5% CO2 incubator at 37 ℃. Luteolin was purchased from Aynor Medicine Technology Co. Ltd (Xi’an, China) at a purity of 98%. Valproic acid was purchased from Sigma-Aldrich Co. (St Louis, MO, USA).
Cell Viability Assay
The three glioma cells in a great condition were seeded in a 96-well plate with 2 × 103 cells per well and incubated overnight at 37 °C. Luteolin was dissolved in DMSO and VPA was dissolved in sterile water at indicated concentrations mixed with 10% FBS-DMEM. The cells were then treated with VPA, luteolin or luteolin + VPA at the indicated concentrations for 24 or 48 h. Subsequently, 10 µL CCK-8 solution (Bimake, Shanghai, China) was added into each well with an incubation for 2 h at 37 ℃ on the shaker. The absorbance value, mostly dominated in the interval from 0.8–1.2, was measured at 450 nm on EL × 800 (BioTek, Winooski, VT, USA) and the control group was considered 100%. The experiment was repeated three times.
Cell Colony Formation Assay
The three glioma cells were cultured into a 12-well plate with 2 × 103 cells per well and incubated overnight at 37 °C. The cells were then treated with VPA, luteolin or luteolin + VPA at the indicated concentrations for 48 h and incubated for consecutive two weeks. The cells were washed with 1 ml PBS each well for three times, fixed with 4% paraformaldehyde for 25 min at 4 ℃ and stained by 0.1% crystal violet for 10 min at room temperature (25 ℃). Subsequently, the plate was washed twice in tap water by dipping into a large beaker and then photographed on a ChemiDoc Touch Imaging System (BioRad, CA, USA). After photograph, each well was treated with 1 ml 1% SDS solution to dissolve stains, placed on the shaker until there is no densely colored area at the bottom of the well. The absorbance value was then detected at 570 nm on EL × 800 (BioTek, Winooski, VT, USA). The experiment was repeated three times.
Cell Wound-Healing Assay
The three glioma cells were seeded in a 12-well plate with 1 × 104 cells per well and incubated overnight at 37 °C. The cells were then treated with VPA, luteolin or luteolin + VPA at the indicated concentrations until they reached > 80% confluence. A 10 µL sterile pipette tip was used to form a scratching wound in the middle of each well, washed by PBS to remove the debris and then cultured with DMEM without FBS. The representative images were taken at 0 h and 24 h on a microscope (IX71, Olympus, Tokyo, Japan). The experiment was repeated three times.
Cell Apoptosis Assay and Cell-Cycle Assay
The three glioma cells were cultured into a 6-well plate with 5 × 104 cells per well and incubated overnight at 37 °C. The cells were then treated with VPA, luteolin or luteolin + VPA at the indicated concentrations for 48 h. For cell apoptosis assay, the cells were trypsinized, washed by PBS twice and resuspended with 1 × Annexin V Binding Buffer (BD Biosciences, USA). The cells were stained with 5 µL of FITC Annexin V and 5 µL of Propidium Iodide Staining Solution for 30 min at room temperature (25 ℃) in the dark and then detected by Guava EasyCyte 6HT-2L flow cytometer (Merck Millipore, Darmstadt, Germany). For cell-cycle assay, the cells were trypsinized, washed by PBS once and fixed in pre-cooled 70% ethanol overnight. The cells were then washed by PBS once, stained with 0.5 mL PI/RNase Staining Buffer (BD Biosciences, USA) per well at room temperature (25 ℃) in the dark and analyzed by Guava EasyCyte 6HT-2L flow cytometer (Merck Millipore, Darmstadt, Germany). The two experiments above were both repeated three times.
Western Blot Analysis
The three glioma cells in a good condition were seeded in a 6-well plate with 5 × 104 cells per well and incubated overnight at 37 ℃. The cells were then treated with VPA, luteolin or luteolin + VPA at the indicated concentrations for 48 h. The cell were washed by PBS twice and lysed in RIPA lysate (Beyotime, Shanghai, China) supplemented with 1% protease and phosphatase inhibitor cocktail (Roche, Basel, Switzerland). The protein samples were the supernatant after centrifugation at 12,000 rcf for 2 min at 4 ℃. The protein concentration was analyzed by a BCA Protein Assay (Thermo Fisher Scientific, MA, USA). The denatured protein samples were distributed through SDS-PAGE, transferred onto polyvinylidene difluoride flat (PVDF) microporous membranes and blocked for 1 h with 5% skimmed milk or Bovine Serum Albumin (BSA). The PVDF membranes were incubated on the shaker at 4 ℃ overnight with the primary antibodies diluted in 5% skimmed milk or BSA: β-actin (#3700), PARP (#9532), Caspase-3 (#9665), cleaved Caspase-3 (#9664), Bax (#2772), Bcl-2 (#2872), p-AKT (#4060), AKT (#4691), p62 (#39,749) and LC3 (#2775). The PVDF membranes were washed by 1 × PBST for 10 min three times, incubated with secondary antibodies for 1 h and washed by 1 × PBST for 10 min three times again. Then the PVDF membranes were detected by using an ECL plus kit (Thermo Fisher Scientific, MA, USA) on a ChemiDoc Touch Imaging System (BioRad, CA, USA) and Chemiluminescent signals were analyzed by Image Laboratory. The experiment was repeated three times.
Statistical Analysis
The results shown were all analyzed from three or more independent and repeated experiments. The data were analyzed by GraphPad Prism 5.0 software and expressed as the mean ± SD. One-way analysis of variance (ANOVA) and two-tailed Student’s t-test were employed to compare different groups. Moreover, *p < 0.05 was considered statistically significant.
Results
VPA Sensitized Glioma Cells to Luteolin in Suppressing Cell Viability
To evaluate the anti-tumor effect of the combination of VPA and luteolin on glioma, the classical glioma cell lines, including U251, LN229 and SNB19, were selected for cytotoxicity tests. The chemical structures of VPA and luteolin are shown in Fig. 1a. The three glioma cells were then treated with VPA or luteolin at the indicated concentrations for 24 or 48 h and the cell viability was detected by Cell Counting Kit-8 (CCK-8) assay. As shown in Fig. 1b, VPA inhibited cell viability in three glioma cells dose- and time-dependently from 2 to 8 mM. Therefore, the VPA concentration of 1 mM and the dosing time of 48 h were chosen for subsequent experiments. Similarly in Fig. 1c, luteolin suppressed cell viability in a dose- and time-dependent manner in the three glioma cells. The half maximal inhibitory concentration (IC50) of luteolin on U251, LN229 and SNB19 at 24 h were 52.11 µM, 46.88 µM and 57.05 µM, while the IC50 were 26.36 µM, 21.48 µM and 23.48 µM at 48 h. Thus, the luteolin concentration of 20 µM and the dosing time of 48 h were selected. Additionally, the cell viability of HEB cells were detected by CCK-8 assay under treatment with VPA-1 mM, Luteolin-20 µM or both for 48 h. As shown in Fig. 1d, the treatment of VPA, luteolin or both at the selected concentrations exerted no inhibitory effects on HEB cells, indicating that the concentration and dosing time could be utilized for cytotoxicity investigations. Moreover, under the treatment with VPA-1 mM and luteolin-20 µM for 48 h, the cell viability of the three glioma cells was increasingly inhibited (Fig. 1e). In conclusion, VPA sensitized glioma cells to luteolin in suppressing cell viability.
VPA sensitized glioma cells to luteolin in suppressing cell viability. a The molecular structural formula of VPA and Luteolin. b The glioma cell lines U251, LN229 and SNB19 cell viability were detected by CCK-8 assay under treatment with the indicated concentrations ranging from 0 to 8 mM of VPA for 24 or 48 h. c The glioma cell lines U251, LN229 and SNB19 cell viability were detected by CCK-8 assay under treatment with the indicated concentrations ranging from 0 to 60 µM of luteolin for 24 or 48 h. d The cell viability of HEB cells were detected by CCK-8 assay under treatment with VPA-1 mM, Luteolin-20 µM or both for 48 h. e The glioma cell lines U251, LN229 and SNB19 cell viability were detected by CCK-8 assay under treatment with VPA-1 mM, Luteolin-20 µM or both for 48 h. The results shown are representatives of three different experiments. **p < 0.01, ***p < 0.001, CCK-8 Cell Counting Kit-8, VPA valproic acid
VPA Sensitized Glioma Cells to Luteolin via Colony Formation and Migration
Cell colony formation assay and cell wound-healing assay were performed to investigate the effect of the sensitization of VPA for luteolin in glioma cells. As shown in Fig. 2a, compared to negative group, luteolin affected the ability of cell colony formation slightly in the three glioma cells, while VPA had no effects on cell colony formation individually. Surprisingly, the combination of VPA and luteolin obtained an exceedingly inhibitory influence on the cellular colony formation. The statistical analysis of cell colony formation assay in the three glioma cells is shown in Fig. 2b. Afterwards, the sensibilization effect of VPA was investigated by cell wound-healing assay. Similarly, though luteolin hindered the speed of wound closure gently and VPA had no respective influence, the combination revealed an enhanced effect in delaying the migration of the three glioma cells (Fig. 2c). The statistical analysis of cell wound healing in the three glioma cells is shown in Fig. 2d. Above all, VPA sensitized glioma cells to luteolin in repressing cellular colony formation and migration.
VPA sensitized glioma cells to luteolin via colony formation and migration. a Representative images of the cell colony formation of the three glioma cell lines U251, LN229 and SNB19 under treatment with VPA-1 mM, Luteolin-20 µM or both for 48 h. b Statistical analysis of colony formation results in the three glioma cell lines. c Representative images of the wound healing of the three glioma cell lines U251, LN229 and SNB19 after treatment with VPA-1 mM, Luteolin-20 µM or both for 48 h. d Statistical analysis of wound-healing results in the three glioma cell lines. The results shown are representatives of three different experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ns no significant, VPA valproic acid
VPA Sensitized Glioma Cells to Luteolin by Induction of Apoptosis and Cell-Cycle Arrest
To explore the effect of the combination of VPA and luteolin on apoptosis and cell-cycle in glioma cells, cell apoptosis assay and cell-cycle assay were applied by flow cytometry. For glioma cell apoptosis, luteolin induced apoptosis slightly in the three glioma cells, while VPA had no obvious effects on cellular apoptosis individually. Astonishingly, the combination of VPA and luteolin induced a higher cellular apoptosis compared to VPA or luteolin, respectively. In U251 cells, the apoptosis rates induced by VPA or luteolin were 9.79% and 11.39%, while the apoptosis rate induced by the combination of VPA and luteolin was 18.01%. In LN229 cells, the apoptosis rates induced by VPA or luteolin were 8.65% and 11.18%, while the apoptosis rate induced by the combination of VPA and luteolin was 23.26%. In SNB19 cells, the apoptosis rates induced by VPA or luteolin were 9.33% and 11.71%, while the apoptosis rate induced by the combination of VPA and luteolin was 17.67% (Fig. 3a). The statistical analysis of cellular apoptosis induced by VPA, luteolin or both is shown in Fig. 3b. For glioma cell-cycle arrest, representative images in Fig. 3c illustrated that VPA potentiated an apparent G0/G1 phase reduction and S and G2/M phase accumulation, while luteolin induced a moderate G0/G1 phase reduction and G2/M phase accumulation in the three glioma cells. Surprisingly, the combination of VPA and luteolin promoted a greater G0/G1 phase reduction and S and G2/M phase accumulation in the three glioma cells. The statistical analysis of cell-cycle arrest induced by luteolin, VPA or both is shown in Fig. 3d.
VPA sensitized glioma cells to luteolin by induction of apoptosis and cell-cycle arrest. a The cell apoptosis of the glioma cell lines U251, LN229 and SNB19 was evaluated by flow cytometry under treatment with VPA-1 mM, Luteolin-20 µM or both for 48 h. b Statistical analysis of cell apoptosis results in the three glioma cell lines. c The cell-cycle stage of the three glioma cell lines U251, LN229 and SNB19 was evaluated by flow cytometry under treatment with VPA-1 mM, Luteolin-20 µM or both for 48 h. d Statistical analysis of cell-cycle results in the three glioma cell lines. The results shown are representatives of three different experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ns no significant, VPA valproic acid
To investigate the underlying mechanism of the combination of VPA and luteolin inducing more glioma cells apoptosis and cell-cycle arrest, western blot analysis was carried out. As shown in Fig. 4a, luteolin promoted a higher expression of cleaved PARP/PARP, cleaved Caspase-3/Caspase-3 and Bax/Bcl-2, while VPA had no effects on apoptosis-related proteins, which was consistent with the apoptotic results. Significantly, the combination of VPA and luteolin induced more increasingly expression of proteins mentioned above than VPA or luteolin individually. The statistical analysis of cleaved PARP/PARP, Bax/Bcl-2 and cleaved Caspase-3/Caspase-3 is shown in Fig. 4b–d. Therefore, all the above results suggested that VPA sensitized glioma cells to luteolin in promoting cell apoptosis and cell-cycle arrest.
VPA sensitized glioma cells to luteolin by elevating expression of apoptosis-related proteins. a The protein levels of PARP, cleaved PARP, Caspase-3, cleaved Caspase-3, Bax and Bcl-2 in the three glioma cell lines U251, LN229 and SNB19 treated with VPA-1 mM, Luteolin-20 µM or both for 48 h were evaluated by western blot analysis. b Statistical analysis of cleaved PARP/PARP in the three glioma cell lines U251, LN229 and SNB19 from western blot results. c Statistical analysis of Bax/Bcl-2 in the three glioma cell lines U251, LN229 and SNB19 from western blot results. d Statistical analysis of cleaved Caspase-3/Caspase-3 in the three glioma cell lines U251, LN229 and SNB19 from western blot results. All the relative protein levels were determined by signal quantification and normalized to β-actin. The results shown are representatives of three different experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ns: no significant, VPA: Valproic acid
VPA Sensitized Glioma Cells to Luteolin Through Autophagy via Akt Signaling
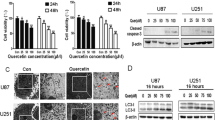
To explore the effect of the combination of VPA and luteolin on autophagy in glioma cells, western blot analysis was carried out to detect the expression level of key autophagy proteins p62 and LC3-II. As shown in Fig. 5a, VPA or luteolin promoted the decrease of p62 and the increase of LC3-II, respectively, which revealed that VPA or luteolin both had an enhanced influence on cellular autophagy. More importantly, the combination of VPA and luteolin induced a lower expression of p62 and a higher expression of LC3-II compared to VPA or luteolin individually. The statistical analysis of p62 and LC3-II is shown in Fig. 5b, c. For further investigation of the potential mechanism of cell autophagy activation, the key protein p-Akt and Akt in Akt signaling pathway was detected by western blot analysis. In Fig. 5a, though the expression of Akt had no obvious fluctuate, VPA or luteolin potentiated the downregulation of p-Akt to varying degrees. Significantly, compared to VPA or luteolin, respectively, the combination of VPA and luteolin downregulated the expression of p-Akt/Akt apparently, which illustrated the activation of Akt signaling pathway, thus promoting cell autophagy (Fig. 5d). Therefore, VPA sensitized glioma cells to luteolin by promoting cell autophagy via Akt signaling pathway.
VPA sensitized glioma cells to luteolin through autophagy via Akt signaling. a The protein levels of p-Akt, Akt, p62, LC3 and β-actin in the three glioma cell lines U251, LN229 and SNB19 were evaluated by western blot analysis. b Statistical analysis of p62 in the three glioma cell lines U251, LN229 and SNB19 from western blot results. c Statistical analysis of LC3-II in the three glioma cell lines U251, LN229 and SNB19 from western blot results. d Statistical analysis of p-Akt/Akt in the three glioma cell lines U251, LN229 and SNB19 from western blot results. All the relative protein levels were determined by signal quantification and normalized to β-actin. The results shown are representatives of three different experiments. *p < 0.05, **p < 0.01, ***p < 0.001, VPA valproic acid
Discussion
The prognosis of patients with gliomas are still far from satisfactory resulting from the undesirable results of the standard chemotherapy for glioma (Xu et al. 2018; Yi et al. 2016). Therefore, it is a clinically urgent demand for novel drugs with high efficiency to be applied into the treatment of gliomas. Luteolin (39, 49, 5, 7‑tetrahydroxyflavone), a flavonoid found in a number of medicinal plants, acted as an anticancer agent against various tumors (Imran et al. 2019; Huang et al. 2019) such as breast cancer (Ahmed et al. 2019), non-small cell lung cancer (Yu et al. 2019) and pancreatic cancer (Li et al. 2018). However, the anti-tumor effects of luteolin on gliomas are still ambiguous. In this study, we confirmed that luteolin exerted inhibitory effects on gliomas by the inhibition of cell viability, cellular colony formation and migration, and the induction of apoptosis and cell-cycle arrest.
Due to its role of dysregulating DNA repair proteins and antagonizing metastasis-associated processes, VPA has been proposed to be involved in the treatment of multiple cancers (Kiweler et al. 2020). In gastric cancer, VPA inhibited cell proliferation via the induction of autophagy targeting HDAC1/2 and HDAC1/PTEN/Akt signaling (Sun et al. 2020). In pancreatic cancer, VPA exhibited anti-tumor activity selectively against EGFR/ErbB2/ErbB3 via induction of ErbB family members-targeting miRNAs (Lin et al. 2019). As in glioma, VPA inhibited proliferation and reduced invasiveness through Wnt/β-Catenin signaling activation (Riva et al. 2018). In this study, we validated the inhibitory effects of VPA on gliomas that VPA inhibited cell viability in a dose- and time-dependent manner. While VPA might function as a chemosensitizer to enhance the anti-tumor effects of basic chemotherapy agents. In MCF-7 breast cancer, VPA and AZD2461 decreased cell viability in a concentration- and time-dependent manner (Sargazi et al. 2019). In pancreatic cancer, combination chemotherapy of VPA and gemcitabine regulated STAT3/Bmi1 pathway to differentially influence the motility (Li et al. 2019). Surprisingly, VPA sensitized temozolomide (TMZ) to glioma cells by downregulating the expression of MGMT (Ryu et al. 2012). In this study, we demonstrated for the first time that VPA sensitized glioma cells to luteolin in suppressing cell viability, cellular colony formation and migration, and inducing apoptosis, cell-cycle arrest and autophagy. Notably, the VPA concentration of 1 mM adopted in this experiment had little cytotoxic effect when used alone.
Apoptosis and autophagy play seminal roles in maintaining organ homeostasis with different biochemical and morphological appearances (Emdad et al. 2019). Apoptosis is a form of programmed cell death that eliminates unwanted or potentially harmful cells (Valentin et al. 2018), which includes two different types of intrinsic and extrinsic pathways and perturbations in endoplasmic reticulum (ER) function (Shirjang et al. 2019). The major actors during the intrinsic apoptosis pathway are the B-cell lymphoma 2 (Bcl-2) protein families including cysteine aspartic acid-specific proteases (caspases), Bcl-2, BCL2-Associated X (Bax) and inhibitor of apoptosis proteins (IAPs) (Curti et al. 2017). In addition, poly ADP-ribose polymerase (PARP) usually acts as a biomarker in the drug-induced apoptosis (Shao et al. 2019). In this study, we found that luteolin could upregulate the protein level of cleaved caspase-3, cleaved PARP and Bax accompanied by the downregulation of Bcl-2, while there is no fluctuate on the expression of Caspase-3 and PARP. Notably, VPA could enhance the sensitization of glioma to luteolin on Caspase-3, PARP, Bax and Bcl-2, indicating the potential of VPA and luteolin to induce more apoptosis in glioma. Autophagy is a self-protecting cellular catabolic pathway, through which some long-lived or misfolded proteins and damaged organelles are degraded into metabolic elements and recycled for the maintenance of cellular homeostasis (Wang et al. 2018). Autophagosomes, which are specialized double-membrane vesicles in autophagic process, deliver cytoplasmic portions into lytic compartments to remove unwanted material or provide energy and biosynthetic building blocks (Ustun et al. 2017). As metrics of autophagy by detecting autophagosomes, a selective recognition receptor p62 and a specific linker LC3-II are usually considered the molecular markers (Song et al. 2012). Moreover, signaling pathways including PI3K/Akt/mTOR and AMPK/TSC/mTOR are usually involved in cellular autophagy (Qin et al. 2010). In this study, the combination treatment of VPA and luteolin remarkably decreased the expression level of p62 and increased the expression level of LC3-II, suggesting the enhanced autophagic response. Furthermore, the VPA treatment led to the obvious downregulation of p-Akt/Akt, suggesting the inhibition of the Akt signaling pathway.
Nevertheless, there are still several limitations with our study. Firstly, more practical detection methods for autophagosomes including transmission electron microscope, fluorescent labeling and MDC staining should be carried out for verification of autophagy induction. Secondly, other key regulators in the PI3K/Akt/mTOR signaling pathway, including PI3K, mTOR, PTEN and PDK, should be evaluated by various biological methods. Thirdly, our results illustrated the induction of apoptosis and autophagy by luteolin and VPA. While the actual protein networks to control regulation and execution of apoptosis and autophagy are highly interconnected (Booth et al. 2019), which needs our further investigation.
In conclusion, our study has demonstrated that VPA sensitized glioma cells to luteolin through induction of apoptosis and autophagy via Akt signaling pathway. A combination therapy of VPA and luteolin may provide a promising therapeutic strategy for glioma in the future.
References
Ahmed S, Khan H, Fratantonio D, Hasan MM, Sharifi S, Fathi N, Ullah H, Rastrelli L (2019) Apoptosis induced by luteolin in breast cancer: Mechanistic and therapeutic perspectives. Phytomedicine 59:152883. https://doi.org/10.1016/j.phymed.2019.152883
Anson DM, Wilcox RM, Huseman ED, Stump TA, Paris RL, Darkwah BO, Lin S, Adegoke AO, Gryka RJ, Jean-Louis DS, Amos S (2018) Luteolin Decreases Epidermal Growth Factor Receptor-Mediated Cell Proliferation and Induces Apoptosis in Glioblastoma Cell Lines. Basic Clin Pharmacol Toxicol 123(6):678–686. https://doi.org/10.1111/bcpt.13077
Booth LA, Roberts JL, Dent P (2019) The role of cell signaling in the crosstalk between autophagy and apoptosis in the regulation of tumor cell survival in response to sorafenib and neratinib. Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2019.10.013
Cao Q, Shi Y, Wang X, Yang J, Mi Y, Zhai G, Zhang M (2019) Circular METRN RNA hsa_circ_0037251 Promotes Glioma Progression by Sponging miR-1229-3p and Regulating mTOR Expression. Sci Rep 9(1):19791. https://doi.org/10.1038/s41598-019-56417-8
Chen JH, Zheng YL, Xu CQ, Gu LZ, Ding ZL, Qin L, Wang Y, Fu R, Wan YF, Hu CP (2017) Valproic acid (VPA) enhances cisplatin sensitivity of non-small cell lung cancer cells via HDAC2 mediated down regulation of ABCA1. Biol Chem 398(7):785–792. https://doi.org/10.1515/hsz-2016-0307
Curti V, Di Lorenzo A, Dacrema M, Xiao J, Nabavi SM, Daglia M (2017) In vitro polyphenol effects on apoptosis: An update of literature data. Semin Cancer Biol 46:119–131. https://doi.org/10.1016/j.semcancer.2017.08.005
Eckert M, Klumpp L, Huber SM (2017) Cellular effects of the antiepileptic drug valproic acid in glioblastoma. Cell Physiol 44(4):1591–1605. https://doi.org/10.1159/000485753
El Gueder D, Maatouk M, Kalboussi Z, Daouefi Z, Chaaban H, Ioannou I, Ghedira K, Ghedira LC, Luis J (2018) Heat processing effect of luteolin on anti-metastasis activity of human glioblastoma cells U87. Environ Sci Pollut Res Int 25(36):36545–36554. https://doi.org/10.1007/s11356-018-3477-x
Emdad L, Bhoopathi P, Talukdar S, Pradhan AK, Sarkar D, Wang XY, Das SK, Fisher PB (2019) Recent insights into apoptosis and toxic autophagy: the roles of MDA-7/IL-24, a multidimensional anti-cancer therapeutic. Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2019.07.013
Gao G, Ge R, Li Y, Liu S (2019) Luteolin exhibits anti-breast cancer property through up-regulating miR-203. Artif Cells Nanomed Biotechnol 47(1):3265–3271. https://doi.org/10.1080/21691401.2019.1646749
Garcia CG, Kahn SA, Geraldo LHM, Romano I, Domith I, Silva D, Dos Santos AF, Ferreira MJ, Portugal CC, de Souza JM, Romao LF, Netto ADP, Lima FRS, Cossenza M (2018) Combination therapy with sulfasalazine and valproic acid promotes human glioblastoma cell death through imbalance of the intracellular oxidative response. Mol Neurobiol 55(8):6816–6833. https://doi.org/10.1007/s12035-018-0895-1
Geng F, Lu GF, Ji MH, Kong DY, Wang SY, Tian H, Xie ZM, Pan M, Gong NL (2019) MicroRNA-26b-3p/ANTXR1 signaling modulates proliferation, migration, and apoptosis of glioma. Am J Transl Res 11(12):7568–7578
Huang L, Jin K, Lan H (2019) Luteolin inhibits cell cycle progression and induces apoptosis of breast cancer cells through downregulation of human telomerase reverse transcriptase. Oncol Lett 17(4):3842–3850. https://doi.org/10.3892/ol.2019.10052
Iida K, Naiki T, Naiki-Ito A, Suzuki S, Kato H, Nozaki S, Nagai T, Etani T, Nagayasu Y, Ando R, Kawai N, Yasui T, Takahashi S (2020) Luteolin suppresses bladder cancer growth via regulation of mechanistic target of rapamycin pathway. Cancer Sci. https://doi.org/10.1111/cas.14334
Imran M, Rauf A, Abu-Izneid T, Nadeem M, Shariati MA, Khan IA, Imran A, Orhan IE, Rizwan M, Atif M, Gondal TA, Mubarak MS (2019) Luteolin, a flavonoid, as an anticancer agent: a review. Biomed Pharmacother 112:108612. https://doi.org/10.1016/j.biopha.2019.108612
Kiweler N, Wunsch D, Wirth M, Mahendrarajah N, Schneider G, Stauber RH, Brenner W, Butter F, Kramer OH (2020) Histone deacetylase inhibitors dysregulate DNA repair proteins and antagonize metastasis-associated processes. J Cancer Res Clin Oncol 146(2):343–356. https://doi.org/10.1007/s00432-019-03118-4
Lee JE, Kim JH (2015) Valproic acid inhibits the invasion of PC3 prostate cancer cells by upregulating the metastasis suppressor protein NDRG1. Genet Mol Biol 38(4):527–533. https://doi.org/10.1590/S1415-475738420150028
Li H, Zhang Z, Gao C, Wu S, Duan Q, Wu H, Wang C, Shen Q, Yin T (2019) Combination chemotherapy of valproic acid (VPA) and gemcitabine regulates STAT3/Bmi1 pathway to differentially potentiate the motility of pancreatic cancer cells. Cell Biosci 9:50. https://doi.org/10.1186/s13578-019-0312-0
Li Z, Zhang Y, Chen L, Li H (2018) The dietary compound luteolin inhibits pancreatic cancer growth by targeting BCL-2. Food Func 9(5):3018–3027. https://doi.org/10.1039/c8fo00033f
Lin T, Ren Q, Zuo W, Jia R, Xie L, Lin R, Zhao H, Chen J, Lei Y, Wang P, Dong H, Huang L, Cai J, Peng Y, Yu Z, Tan J, Wang S (2019) Valproic acid exhibits anti-tumor activity selectively against EGFR/ErbB2/ErbB3-coexpressing pancreatic cancer via induction of ErbB family members-targeting microRNAs. J Exp Clin Cancer Res CR 38(1):150. https://doi.org/10.1186/s13046-019-1160-9
Naiki-Ito A, Naiki T, Kato H, Iida K, Etani T, Nagayasu Y, Suzuki S, Yamashita Y, Inaguma S, Onishi M, Tanaka Y, Yasui T, Takahashi S (2019) Recruitment of miR-8080 by luteolin inhibits androgen receptor splice variant 7 expression in castration-resistant prostate cancer. Carcinogenesis. https://doi.org/10.1093/carcin/bgz193
Qin L, Wang Z, Tao L, Wang Y (2010) ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy 6(2):239–247. https://doi.org/10.4161/auto.6.2.11062
Riva G, Cilibrasi C, Bazzoni R, Cadamuro M, Negroni C, Butta V, Strazzabosco M, Dalpra L, Lavitrano M, Bentivegna A (2018) Valproic acid inhibits proliferation and reduces invasiveness in glioma stem cells through Wnt/beta catenin signalling activation. Genes. https://doi.org/10.3390/genes9110522
Ryu CH, Yoon WS, Park KY, Kim SM, Lim JY, Woo JS, Jeong CH, Hou Y, Jeun SS (2012) Valproic acid downregulates the expression of MGMT and sensitizes temozolomide-resistant glioma cells. J Biomed Biotechnol 2012:987495. https://doi.org/10.1155/2012/987495
Sargazi S, Kooshkaki O, Zavar Reza J, Saravani R, Zarei Jaliani H, Mirinejad S, Meshkini F (2019) Mild antagonistic effect of Valproic acid in combination with AZD2461 in MCF-7 breast cancer cells. Med J Islamic Repub Iran 33:29. https://doi.org/10.34171/mjiri.33.29
Shao N, Mao J, Xue L, Wang R, Zhi F, Lan Q (2019) Carnosic acid potentiates the anticancer effect of temozolomide by inducing apoptosis and autophagy in glioma. J Neurooncol 141(2):277–288. https://doi.org/10.1007/s11060-018-03043-5
Shirjang S, Mansoori B, Asghari S, Duijf PHG, Mohammadi A, Gjerstorff M, Baradaran B (2019) MicroRNAs in cancer cell death pathways: Apoptosis and necroptosis. Free Radical Biol Med 139:1–15. https://doi.org/10.1016/j.freeradbiomed.2019.05.017
Song CY, Guo JF, Liu Y, Tang BS (2012) Autophagy and its comprehensive impact on ALS. Int J Neurosci 122(12):695–703. https://doi.org/10.3109/00207454.2012.714430
Sun J, Piao J, Li N, Yang Y, Kim KY, Lin Z (2020) Valproic acid targets HDAC1/2 and HDAC1/PTEN/Akt signalling to inhibit cell proliferation via the induction of autophagy in gastric cancer. FEBS J 287(10):2118–2133. https://doi.org/10.1111/febs.15122
Tseng JH, Chen CY, Chen PC, Hsiao SH, Fan CC, Liang YC, Chen CP (2017) Valproic acid inhibits glioblastoma multiforme cell growth via paraoxonase 2 expression. Oncotarget 8(9):14666–14679. https://doi.org/10.18632/oncotarget.14716
Ustun S, Hafren A, Hofius D (2017) Autophagy as a mediator of life and death in plants. Curr Opin Plant Biol 40:122–130. https://doi.org/10.1016/j.pbi.2017.08.011
Valentin R, Grabow S, Davids MS (2018) The rise of apoptosis: targeting apoptosis in hematologic malignancies. Blood 132(12):1248–1264. https://doi.org/10.1182/blood-2018-02-791350
Wang P, Shao BZ, Deng Z, Chen S, Yue Z, Miao CY (2018) Autophagy in ischemic stroke. Prog Neurobiol 163–164:98–117. https://doi.org/10.1016/j.pneurobio.2018.01.001
Wu C, Xu Q, Chen X, Liu J (2019) Delivery luteolin with folacin-modified nanoparticle for glioma therapy. Int J Nanomed 14:7515–7531. https://doi.org/10.2147/IJN.S214585
Wu L, Feng H, Hu J, Tian X, Zhang C (2016) Valproic acid (VPA) promotes the epithelial mesenchymal transition of hepatocarcinoma cells via transcriptional and post-transcriptional up regulation of Snail. Biomed Pharmacother 84:1029–1035. https://doi.org/10.1016/j.biopha.2016.10.023
Xiong Z, Zhou C, Wang L, Zhu R, Zhong L, Wan D, Wang Q (2019) Circular RNA SMO sponges miR-338-3p to promote the growth of glioma by enhancing the expression of SMO. Aging (Albany NY) 11(24):12345–12360. https://doi.org/10.18632/aging.102576
Xu X, Wang Z, Liu N, Cheng Y, Jin W, Zhang P, Wang X, Yang H, Liu H, Zhang Y, Tu Y (2018) Association between SOX9 and CA9 in glioma, and its effects on chemosensitivity to TMZ. Int J Oncol 53(1):189–202. https://doi.org/10.3892/ijo.2018.4382
Xu Y, Xu D, Zhu SJ, Ye B, Dong JD, Zhang YL, Zhang Y (2015) Induction of apoptosis and autophagy in metastatic thyroid cancer cells by valproic acid (VPA). Int J Clin Exp Pathol 8(7):8291–8297
Yao Y, Rao C, Zheng G, Wang S (2019) Luteolin suppresses colorectal cancer cell metastasis via regulation of the miR384/pleiotrophin axis. Oncol Rep 42(1):131–141. https://doi.org/10.3892/or.2019.7136
Yi GZ, Liu YW, Xiang W, Wang H, Chen ZY, Xie SD, Qi ST (2016) Akt and beta-catenin contribute to TMZ resistance and EMT of MGMT negative malignant glioma cell line. J Neurol Sci 367:101–106. https://doi.org/10.1016/j.jns.2016.05.054
You Y, Wang R, Shao N, Zhi F, Yang Y (2019) Luteolin suppresses tumor proliferation through inducing apoptosis and autophagy via MAPK activation in glioma. Onco Targets Ther 12:2383–2396. https://doi.org/10.2147/OTT.S191158
Yu Q, Zhang M, Ying Q, Xie X, Yue S, Tong B, Wei Q, Bai Z, Ma L (2019) Decrease of AIM2 mediated by luteolin contributes to non-small cell lung cancer treatment. Cell Death Dis 10(3):218. https://doi.org/10.1038/s41419-019-1447-y
Zhang C, Liu S, Yuan X, Hu Z, Li H, Wu M, Yuan J, Zhao Z, Su J, Wang X, Liao Y, Liu Q (2016) Valproic acid promotes human glioma U87 cells apoptosis and inhibits glycogen synthase kinase-3beta through ERK/Akt signaling. Cell Physiol Biochem 39(6):2173–2185. https://doi.org/10.1159/000447912
Zheng S, Cheng Y, Teng Y, Liu X, Yu T, Wang Y, Liu J, Hu Y, Wu C, Wang X, Liu Y, You C, Gao X, Wei Y (2017) Application of luteolin nanomicelles anti-glioma effect with improvement in vitro and in vivo. Oncotarget 8(37):61146–61162. https://doi.org/10.18632/oncotarget.18019
Funding
No financial support.
Author information
Authors and Affiliations
Contributions
HW, YF and WR are responsible for the conceptualization and design of the studies. HW and WR performed all experiments and analyzed the data. HW and YF wrote the manuscript. GW and ZF reviewed and finalized the final draft.
Corresponding authors
Ethics declarations
Conflict of interest:
All the authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Han, W., Yu, F., Wang, R. et al. Valproic Acid Sensitizes Glioma Cells to Luteolin Through Induction of Apoptosis and Autophagy via Akt Signaling. Cell Mol Neurobiol 41, 1625–1634 (2021). https://doi.org/10.1007/s10571-020-00930-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-020-00930-2