Abstract
Doxycycline (Dox)-inducible transgenic approach is used to examine the neural mechanisms of anxiety and depression; however, its own effects on related behaviors are not clear. To address this, in the present study, we tested the anxiety- and depression-like behaviors in rats treated with Dox in drinking water (2 mg/ml) in the elevated plus-maze (EPM; on day 5) and forced swim (FST; on day 8) tests, respectively. In addition, the levels of mRNAs and proteins of brain-derived neurotrophic factor (BDNF) and anti-apoptotic protein Bcl-xL in the hippocampus (HIPP) and frontal cortex (FC) were also analyzed. Consumption of Dox for 4 days induced an anxiogenic-like phenotype that was manifested by the decreased percentages of open arm entries and time spent on the open arms of the EPM. After Dox for 7 days, animals demonstrated more active behavior in the FST than control rats as evidenced by the increase in climbing time. When assessed after the FST, expression of Bcl-xL was increased in the hippocampus of Dox-treated animals. Furthermore, hippocampal Bcl-xL content correlated positively with the duration of climbing in the test. This study is the first to find that Dox in treatment regime used to control transgene expression can affect anxiety- and depression-like behaviors in rats. Dox-induced increase in Bcl-xL expression in the hippocampus may be involved in the moderate activation of FST behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Doxycycline (Dox), the tetracycline antibiotic with a good penetration into the brain (Barza et al. 1975; Yim et al. 1985), is widely used for the control of transgene expression in studies of brain physiology (Chen et al. 2014; Barroso-Chinea et al. 2016) and behavior (Chandrasekar and Dreyer 2011; Fitting et al. 2013; Paris et al. 2014; Vaisburd et al. 2015; Hahn et al. 2016). To modulate gene expression in rodents, Dox is usually administered with chow (Chtarto et al. 2016; Hahn et al. 2016) or in drinking water (Chen et al. 2014; Chiken et al. 2015; Vaisburd et al. 2015; Barroso-Chinea et al. 2016) for several days or weeks and, more rarely, by intraperitoneal injections (Paris et al. 2014; Kesby et al. 2016, 2017).There are, however, several observations that Dox at the doses and routes commonly used in these studies has its own effects that may confound interpretation of changes seen after modulation of target gene expression. For example, Dox protected the dopaminergic neurons in the model of Parkinson’s disease (Lazzarini et al. 2013), activated adult neurogenesis (Sultan et al. 2013), and reduced alcohol consumption (McIver et al. 2012).
Despite that the Dox-controlled gene expression systems are used in investigations of the neural mechanisms associated with anxiety (Paris et al. 2014; Vaisburd et al. 2015; Hahn et al. 2016) and depression (Vicentini et al. 2009; Serchov et al. 2015), it is not clear whether Dox influences related behaviors. The psycho-emotional effects of Dox have received much less attention than, for example, the other tetracycline antibiotic, minocycline (Levkovitz et al. 2015; Vogt et al. 2016), that was even suggested as a potential antidepressant drug (Soczynska et al. 2012). There are few ongoing clinical and experimental studies. Thus, clinical descriptions of patients indicate the occurrence of psychiatric side effects, including anxiety, depression, and even suicide attempts, after treatment with Dox (Atigari et al. 2013). On the other hand, the patients with neuropsychiatric disturbances during Lyme neuroborreliosis (Markeljević et al. 2011; Nagy et al. 2016) or neurobrucellosis (Tekin-Koruk et al. 2010) responded by rapid functional improvement to therapy with Dox combined with other antibiotics. The attenuation of the recall of threat memory in individuals who were under Dox during acquisition indicates the potential significance of Dox for prevention and treatment of anxiety (Bach et al. 2017). In animal studies, Dox administered to mice for 2 (Paris et al. 2014) or 8 (Vaisburd et al. 2015) weeks had no effects on anxiety-related behaviors. And in fact, there is only one study, in which depression-like behavior was assessed in rats after a single Dox injection (Mello et al. 2013).
Given the extensive use of Dox to control transgene expression, the need to further evaluate drug own functional effects is evident. In the present study, we tested anxiety- and depression-like behaviors in the same rats at two time points (4th and 7th days) of Dox administration with drinking water. In addition, because brain-derived neurotrophic factor (BDNF) (Duman and Monteggia 2006) and anti-apoptotic proteins (McKernan et al. 2009) were implicated in pro- and antidepressant effects of various treatments, and little is known about effects of Dox on these parameters (Mello et al. 2013), expression of BDNF and Bcl-xL in the hippocampus and frontal cortex was also assessed.
Materials and Methods
Animals and Experimental Design
Adult male Wistar rats of two groups (11–12 animals per group) weighing 273 ± 7 and 271 ± 10 g were used in the experiments. All animal use procedures were supervised and specifically approved by the ethic committee of the Institute of Cytology and Genetics in accordance with the guidelines of the Ministry of Public Health of Russia (supplement to order N 267 of June 19, 2003) and the European Council Directive (86/609/EEC). All efforts were made to minimize animal suffering and to reduce the number of animals used.
To control transgene expression, Dox is usually administered per os with food or drinking water. Therefore in our experiment, animals of the first group received drinking water supplemented with 5% sucrose containing 2 mg/ml Dox (JSC “Sintez,” Russia) for 7 days, whereas the animals of the second (control) group received normal drinking water. As was shown in study of Liu et al. (2008), such treatment regime with Dox completely repressed transgene expression in the rat brain. In experiments in vitro, similar inhibition of target gene expression was observed in cells after Dox for 2 days.
After 4 and 7 days of Dox administration, anxiety- and depression-like behaviors were assessed in the same animals in the elevated plus-maze (EPM) test and the forced swim test (FST), respectively. Thirty minutes after the second swim session, rats were killed by rapid decapitation. The hippocampus (HIPP) and frontal cortex (FC) were immediately dissected out on a cooled plate and frozen in liquid nitrogen for assessment of BDNF and Bcl-xL mRNA and protein levels by real-time PCR and immunoblotting, respectively.
Elevated Plus-Maze (EPM) Test
On the fifth day of Dox administration, the elevated plus-maze test (Pellow et al. 1985; Walf and Frye 2007) was performed between 14:00 and 16:00 p.m. The maze elevated 65 cm above the floor was consisted of two opposite open arms (45 × 10 cm2) and two opposite enclosed arms (45 × 10 × 40 cm3). All four arms were connected to a 10 × 10 cm2 center square. Before the next rat was introduced, the maze was cleaned with a water-wet sponge and then dried. The behavior of the animals was recorded on video for later analysis. Each rat was placed in the center of the maze facing an open arm. During the 5-min test, standard measures such as percentage of entries into and time spent in the open arms, entries into and time spent in the closed arms, and total arm entries were quantified. Arm entry defined when all four paws were on the arm. In addition, the numbers of rearing (rising on the hind limbs) in the closed arms, head-dipping from open arms, and risk-assessment (included the stretched-attend posture and closed arm returns) were also analyzed.
Forced Swim Test (FST)
The two-day FST (Porsolt et al. 1978; Slattery and Cryan 2012) was conducted, starting from the third day after the EPM test. On the seventh day of Dox administration, all rats were forced to a pretest swim for 15 min followed 24 h later by a 5-min test. Both swim sessions were carried out between 1000 and 1200 h by placing rats in the cylinder (46 cm high, 20 cm in diameter) filled with water (25 °C) to a height of 30 cm. Animal behaviors were recorded on videotape, and the latency to the first immobility and total duration of immobility during the first 5-min pretest and test sessions were measured later. In the test session, swimming time and climbing time were additionally assessed (Detke and Lucki 1996; Cryan et al. 2002). After swimming, the rats were dried with towels and returned to their home cages. The swim cylinders were cleaned, and the water was exchanged between rats.
Analysis of Messenger RNAs
Total RNA was isolated from the brain tissue samples by a one-step guanidine isothiocyanate method. The mRNAs for the bdnf and bclxL genes were quantified by real-time qPCR with the actb mRNA as a calibrator using a set of TaqMan® Gene Expression Assay primers/probes (Rn02531967_s1 for bdnf, Rn00437783_m1 for bclxL, and Rn00667869_m1 for actb; Applied Biosystems, USA) in an ABI VIIA™ 7 Real-Time PCR System (Applied Biosystems). All reactions were carried out in duplicate on cDNA samples in 96-well optical plates according to the manufacturer’s protocol in 25 μl of 1× TaqMan1Universal PCR MasterMix (Applied Biosystems). The real-time PCR consisted of one cycle of 50 °C for 2 min and 95 °C for 10 min, followed by 40 cycles each of 95 °C for 15 s and 60 °C for 1 min. The comparative ΔΔCT method was used to calculate mRNA expression relative to the beta-actin as an endogenous control according to manufacturer’s manual (Applied Biosystems).
Western Blotting Analysis
To estimate the levels of Bcl-xL protein by Western blot analysis, hippocampal and cortical tissue samples were homogenized in lysis buffer containing 150 mM NaCl, 50 mM Tris, 1% Triton X100, and protease inhibitors (2 mM PMSF, 2 μg/ml leupeptin, 2 μg/ml pepstatin, and 2 μg/ml aprotinin). Protein samples (50 μg) were fractionated by electrophoresis in 12% polyacrylamide gel with sodium dodecyl sulfate in a Mini-Protean 3 Dodeca Cell (Bio-Rad, USA) and transferred onto 0.45-μm nitrocellulose membrane with a Trans-Blot system (Bio-Rad). The proteins were stained with primary anti-Bcl-xL or BDNF rabbit monoclonal antibodies (dilution 1:500; Cell Signaling, USA) and rabbit polyclonal anti-β-actin antibodies (dilution 1:20,000; Santa Cruz Biotechnology, USA; sc1616). Secondary anti-rabbit IgG (Bio-Rad) were used at 1:1000 dilutions for BDNF and Bcl-xL staining, and 1:10,000 dilution for β-actin staining. The blots were developed with a SuperSignal™ West Femto Maximum Sensitivity Substrate chemiluminescence kit (Life Technologies, USA) and quantified after scanning with a ChemidocTM Touch Imaging System (Bio-Rad) using the Scion Image 4.0.3.2 program (Scion Corporation, USA). The amounts of Bcl-xL and BDNF proteins were expressed in arbitrary units relatively to the amounts of β-actin in the same sample.
Statistical Analysis
The effects of Dox on behaviors and neurobiological parameters were analyzed by comparing Dox-treated and untreated groups using a Student’s t test. In an attempt to dissociate locomotor activity from anxiety in the elevated plus-maze test, analysis of covariance was performed with open arm entries as the dependent variable, and total arm entries as the covariate. Two-way repeated measures ANOVA was used to analyze the differences between the behaviors of animals in pretest and test swim sessions. Pearson’s correlation analysis on the data from both Dox-treated and untreated groups was used to evaluate relationships between parameters. The results were considered significant at a probability level of less than 0.05.
Results
Effects of Dox on Behaviors of Rats in the EPM Test
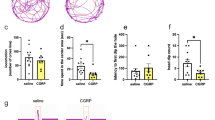
As shown in Fig. 1, animals treated with Dox for 4 days had a reduced number of entries into the open arms (Fig. 1a, t = 3.446, df = 21, P = 0.002) and spent less time (Fig. 1b, t = 3.242, df = 21, P = 0.004) on open arms than control rats. Evidenced by these changes, an anxiogenic-like effect of Dox is in accordance with the alterations in ethological parameters associated with anxiety state. Dox-treated animals showed a significant decrease in head-dipping (untreated: 4.00 ± 0.88, Dox-treated: 0.91 ± 0.62; t = 2.819, df = 21, P = 0.010) and a trend of increase in risk-assessment (untreated: 2.25 ± 0.63, Dox-treated: 3.82 ± 0.46; t = 1.976, df = 21, P = 0.061). The total number of entries suggested as a mixed index related to both anxiety and motor activity (Cruz et al. 1994) was decreased in Dox-treated animals (Fig. 1c, t = 3.273, df = 21, P = 0.002). Analysis of covariance showed that a decrease in the open arms entries was dependent on the decrease in total arm entries [F(1,20) = 1.647, P = 0.214]. No effect of Dox on the number of rears (Fig. 1d, t = 0.675, df = 21, P = 0.507), which was related only to general activity of rats (Cruz et al. 1994), diminished the possible dependence of Dox-induced decrease in open arm activity on inhibited locomotion.
Effects of Dox on Behaviors of Rats in the FST
The rat FST includes the pretest session needed for inducing a high level of immobility, which, especially in subsequent test session, is sensitive to pro- and antidepressant drugs (Porsolt et al. 1978). Another parameter used to assess an antidepressant-like activity is the latency to immobility that was shown to improve the sensitivity of the FST to some antidepressants (Castagné et al. 2009). Because treatment of rats with Dox was started prior to pretest that, as was suggested, could potentially alter behavior in this and therefore in the subsequent test session (Slattery and Cryan 2012), the latency to immobility and immobility time were analyzed during the first 5 min in both pretest and test sessions. The values of these parameters in the pretest were not significantly affected by Dox (latency to immobility, s: control—112.2 ± 10.5, Dox—108.2 ± 9.1, t = 0.284, df = 21, P = 0.779; immobility time, s: control—128.3 ± 9.5, Dox—139.5 ± 11.6, t = 0.762, df = 21, P = 0.455). As expected, all control and Dox-treated rats displayed longer immobility time in the test than in the pretest [F(1,21) = 18.355, P < 0.001] (data not shown). However, in contrast to control rats, the animals treated with Dox did not show an expected decrease in latency to immobility from pretest to test sessions [F(1,21) = 2.853, P = 0.105] (data not shown).
Analysis of the effects of Dox on behaviors in the test session showed a tendency to increased latency to immobility in Dox-treated animals as compared to controls (t = 1.845, df = 21, P = 0.079) (Fig. 2), which may result from a more active behavior after Dox. Such possibility is supported by data showing that although Dox did not affect the duration of immobility or swimming, the significant increase in the duration of climbing (t = 2.082, df = 21, P = 0.049) was observed in the test session after the drug.
The ffects of Dox (2 mg/ml in drinking water for 7 days) on behaviors in the FST: immobility latency (Immobility L), immobility time (Immobility), swimming time (Swimming), and climbing time (Climbing). Data normalized to control group as 100% are expressed as mean ± SEM. (n = 11–12). *P < 0.05 compared to appropriate control group
Analysis of Correlations Between Behavioral Parameters from EPM and FST
Behavior in the FST may be confounded by changes in locomotor activity (Slattery and Cryan 2012). Possible interrelations of Dox effects on behaviors in the EPM test and FST were evaluated by estimation of correlations between behavioral measures from these tests (Pearson’s test; n = 23). No significant correlations between the latency to immobility or climbing time in the FST and the EPM behaviors such as the number of open arm entries (r = −0.25 and r = −0.26, respectively), the number of closed entries (r = −0.11 and r = −0.08), total number of arm entries (r = −0.18 and r = −0.16), number of rears (r = 0.01 and r = −0.02), head-dips (r = −0.11 and r = 0.03), or risk-assessment (r = 0.33 and r = 0.33) were observed.
Effects of Dox on Brain BDNF and Bcl-xL Expression
The results of real-time PCR and immunoblot analysis (Fig. 3a, b) show that Dox administration for 7 days did not affect BDNF mRNA and protein levels in the HIPP and FC. In contrast to BDNF, Bcl-xL mRNA levels were increased by Dox in the HIPP (t = 2.752, df = 11, P = 0.018), but not in the FC. The slight increase in the HIPP Bcl-xL protein levels after Dox was not significant (t = 1.574, df = 13, P = 0.245). No changes in Bcl-xL protein levels were observed in the FC.
Analysis of Correlations Between Neurobiological and FST Behavioral Parameters
A significant positive correlation was revealed between the protein levels of Bcl-xL in the hippocampus and duration of climbing in the FST (Fig. 4; r = 0.71, P = 0.003, n = 15). No significant correlations were identified between neurobiological parameters investigated and immobility latency, duration of immobility, or swimming (data not shown).
Discussion
Here, we show, for the first time, that Dox in treatment regime used to control transgene expression can affect anxiety- and depression-like behaviors in rats and Bcl-xL expression in their hippocampus.
First, Dox for 4 days significantly enhanced anxiety as evidenced by the decreased percentages of open arm entries and time spent on the open arms. It is important to highlight that although Dox decreased total arm entries suggested as a measure of spontaneous motor activity in the plus-maze (Walf and Frye 2007), no changes in rearing, the other indicator of locomotor activity, together with the decrease in head-dipping and moderate elevation in risk-assessment allow thinking that Dox rather affected anxiety than locomotor activity. The absence of differences in locomotor activity between WT mice that consumed Dox with drinking water (2 mg/ml) for 7–28 days and untreated D1RKD mice (Chiken et al. 2015) could be viewed as supporting evidence that Dox has no effects on locomotion. The observed anxiogenic-like effect of Dox is in agreement with the finding that anxiety was listed as a side effect of Dox in the British National Formulary (Atigari et al. 2013). However, in a mutant mouse model of schizophrenia, Vaisburd and colleagues (Vaisburd et al., 2015) investigated anxiety following long-term Dox treatment (2 mg/ml in drinking water for 8 weeks), but the effects of Dox on such measure of anxious behavior as the time spent on the closed arms of the EPM were not directly shown in both mutant and control mice of wild type. Similarly, no significant effects of Dox (100 mg/kg, i.p.) for 14 days on anxiety-like behavior have been reported in C57BL/6J mice in the open field, social interaction, and marble burying tests (Paris et al. 2014). The differences in Dox effects on anxiety among studies may be explained, for example, by different durations of the drug administration.
Second, in the FST test session, the latency to the first immobility in Dox-treated animals tended to be longer than in control rats indicating a trend toward more active behavior in the FST that can be interpreted as a moderate antidepressant-like effect. The increase in climbing time in the FST supports the suggestion that Dox-treated rats really exerted more effort to escape from the stressful conditions of the test. Trend toward increased antidepressant-like activity observed after Dox is in agreement with in fact a single experimental study, in which the effect of Dox on depression-like behavior in animals was evaluated (Mello et al. 2013). In this study, Dox administered 30 min before lipopolysaccharide (LPS) prevented LPS-induced immobility in the FST. Moreover, Dox significantly decreased immobility time when compared not only to LPS-treated mice, but also to saline-treated controls, thereby indicating a clear antidepressant-like effect. One possible explanation for more evident Dox effect in LPS-induced model of depression than in our study is that LPS causes activation of inflammatory system that is associated with depression (Stepanichev et al. 2014) and anti-inflammatory properties of Dox supported in the study of Mello et al. (2013) by the decreases in IL-1beta levels in some brain regions.
Third, at 30 min after the FST exposure, Dox-treated rats demonstrated an increase in Bcl-xL mRNA expression in the hippocampus as compared to control animals. Moreover, despite no changes in Bcl-xL protein levels, a significant positive correlation was observed between the levels of hippocampal Bcl-xL protein and climbing time in the FST. Based on the data of the involvement of anti-apoptotic proteins, including Bcl-xL, in supplying of brain cells with energy (Du et al. 2009; Jonas et al. 2014), it could be speculated that the more active behavior of Dox-treated rats in the FST may be at least partly resulted from the activation of Bcl-xL expression. This suggestion is in agreement with previous reports, showing the increase in the hippocampal Bcl-xL expression in parallel with the amelioration of the chronic stress-induced depression-like state in rats by swimming exercise (Jiang et al. 2014) and the involvement of the increase in Bcl-xL expression in the rat hippocampus under stress in resilience to stress-induced depression (Shishkina et al. 2010; Dygalo et al. 2012).
BDNF has been shown to play an important role in mediating the therapeutic effects of antidepressants (Adachi et al. 2008). Possible implication of BDNF in antidepressant-like effects of Dox is supported by two studies. In the first study (Mello et al. 2013), an antidepressant-like effect of a single Dox injection in LPS-induced model of depression was accompanied by the increase in the hippocampal BDNF expression. In the second study, 4 weeks of treatment of mice with Dox in food pellets at doses used to control transgene expression resulted in increased neurogenesis (Sultan et al. 2013). Activation of hippocampal neurogenesis that is regulated in part by BDNF (Scharfman et al. 2005) has been associated with the effects of antidepressants (Malberg et al. 2000). However, in our study, we found no evidence for an influence of Dox for 7 days on BDNF expression in the hippocampus. It is possible that more prolonged Dox administration required for enhancing the expression of the neurotrophic factor and producing a sustainable antidepressant effect.
Changes in climbing behavior in the FST (Detke and Lucki 1996; Cryan et al. 2002) indicate that mechanisms of a Dox antidepressant-like action may involve impact on the brain catecholaminergic systems, for example, dopaminergic (DA) activity. It was shown that in transgenic mice with Dox-induced TAT protein expression in the brain, the associated dysfunction in the brain DA systems that include the decrease in dopamine levels (Kesby et al. 2016) and receptor expression (DRD1a, DRD2, DRD4, and DRD5) (Kesby et al. 2017) in the nucleus accumbens resulted in appearance of such key feature of depression as impairments in the response to pleasure (Kesby et al. 2016). On the other hand, Dox can protect the brain DA neurons (Lazzarini et al. 2013; Zhang et al. 2015). As was shown on the different models of Parkinson’s disease, Dox increased the content of dopamine (Zhang et al. 2015) and tyrosine hydroxylase (Lazzarini et al. 2013) in the striatum.
In overall, the data that Dox moderately increased the length of the first effort to escape from the stressful conditions of the forced swim test provide additional evidence in support of the antidepressant-like potential of Dox. However, further investigations are needed to clarify this issue.
Conclusions
Dox in treatment regime used to control of transgene expression has its own effects on behaviors in the EPM and FST, and expression of Bcl-xL in the hippocampus.
References
Adachi M, Barrot M, Autry AE, Theobald D, Monteggia LM (2008) Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol Psychiatry 63(7):642–649
Atigari OV, Hogan C, Healy D (2013) Doxycycline and suicidality. BMJ Case Rep. doi:10.1136/bcr-2013-200723
Bach DR, Tzovara A, Vunder J (2017) Blocking human fear memory with the matrix metalloproteinase inhibitor doxycycline. Mol Psychiatry. doi:10.1038/mp.2017.65
Barroso-Chinea P, Cruz-Muros I, Afonso-Oramas D, Castro-Hernández J, Salas-Hernández J, Chtarto A, Luis-Ravelo D, Humbert-Claude M, Tenenbaum L, González-Hernández T (2016) Long-term controlled GDNF over-expression reduces dopamine transporter activity without affecting tyrosine hydroxylase expression in the rat mesostriatal system. Neurobiol Dis 88:44–54. doi:10.1016/j.nbd.2016.01.002
Barza M, Brown RB, Shanks C, Gamble C, Weinstein L (1975) Relation between lipophilicity and pharmacological behavior of minocycline, doxycycline, tetracycline, and oxytetracycline in dogs. Antimicrob Agents Chemother 8(6):713–720
Castagné V, Porsolt RD, Moser P (2009) Use of latency to immobility improves detection of antidepressant-like activity in the behavioral despair test in the mouse. Eur J Pharmacol 616(1–3):128–133. doi:10.1016/j.ejphar.2009.06.018
Chandrasekar V, Dreyer JL (2011) Regulation of MiR-124, Let-7d, and MiR-181a in the accumbens affects the expression, extinction, and reinstatement of cocaine-induced conditioned place preference. Neuropsychopharmacology 36(6):1149–1164. doi:10.1038/npp.2010.250
Chen SS, Yang C, Hao F, Li C, Lu T, Zhao LR, Duan WM (2014) Intrastriatal GDNF gene transfer by inducible lentivirus vectors protects dopaminergic neurons in a rat model of parkinsonism. Exp Neurol 261:87–96. doi:10.1016/j.expneurol.2014.06.022
Chiken S, Sato A, Ohta C, Kurokawa M, Arai S, Maeshima J, Sunayama-Morita T, Sasaoka T, Nambu A (2015) Dopamine D1 receptor-mediated transmission maintains information flow through the cortico-striato-entopeduncular direct pathway to release movements. Cereb Cortex 25(12):4885–4897. doi:10.1093/cercor/bhv209
Chtarto A, Humbert-Claude M, Bockstael O, Das AT, Boutry S, Breger LS, Klaver B, Melas C, Barroso-Chinea P, Gonzalez-Hernandez T, Muller RN, DeWitte O, Levivier M, Lundberg C, Berkhout B, Tenenbaum L (2016) A regulatable AAV vector mediating GDNF biological effects at clinically-approved sub-antimicrobial doxycycline doses. Mol Ther Methods Clin Dev 5:16027. doi:10.1038/mtm.2016.27
Cruz APM, Frei F, Graeff FG (1994) Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav 49:171–176
Cryan JF, Page ME, Lucki I (2002) Noradrenergic lesions differentially alter the antidepressant-like effects of reboxetine in a modified forced swim test. Eur J Pharmacol 436(3):197–205
Detke MJ, Lucki I (1996) Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behav Brain Res 73(1–2):43–46
Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, Falke C, Khairova R, Zhou R, Yuan P, Machado-Vieira R, McEwen BS, Manji HK (2009) Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci USA 106(9):3543–3548. doi:10.1073/pnas.0812671106
Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59(12):1116–1127. doi:10.1016/j.biopsych.2006.02.013
Dygalo NN, Kalinina TS, Bulygina VV, Shishkina GT (2012) Increased expression of the anti-apoptotic protein Bcl-xL in the brain is associated with resilience to stress-induced depression-like behavior. Cell Mol Neurobiol 32(5):767–776. doi:10.1007/s10571-011-9794-y
Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lichtman AH, Wise LE, Fox MA, Su J, Medina AE, Krahe TE, Knapp PE, Guido W, Hauser KF (2013) Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol Psychiatry 73(5):443–453. doi:10.1016/j.biopsych.2012.09.026
Hahn YK, Paris JJ, Lichtman AH, Hauser KF, Sim-Selley LJ, Selley DE, Knapp PE (2016) Central HIV-1 Tat exposure elevates anxiety and fear conditioned responses of male mice concurrent with altered mu-opioid receptor-mediated G-protein activation and β-arrestin 2 activity in the forebrain. Neurobiol Dis 92:124–136. doi:10.1016/j.nbd.2016.01.014
Jiang P, Dang RL, Li HD, Zhang LH, Zhu WY, Xue Y, Tang MM (2014) The impacts of swimming exercise on hippocampal expression of neurotrophic factors in rats exposed to chronic unpredictable mild stress. Evid Based Complement Altern Med 2014:729827. doi:10.1155/2014/729827
Jonas EA, Porter GA, Alavian KN (2014) Bcl-xL in neuroprotection and plasticity. Front Physiol 5:355. doi:10.3389/fphys.2014.00355
Kesby JP, Markou A, Semenova S (2016) The effects of HIV-1 regulatory TAT protein expression on brain reward function, response to psychostimulants and delay-dependent memory in mice. Neuropharmacology 109:205–215. doi:10.1016/j.neuropharm.2016.06.011
Kesby JP, Najera JA, Romoli B, Fang Y, Basova L, Birmingham A, Marcondes MCG, Dulcis D, Semenova S (2017) HIV-1 TAT protein enhances sensitization to methamphetamine by affecting dopaminergic function. Brain Behav Immun. doi:10.1016/j.bbi.2017.05.004
Lazzarini M, Martin S, Mitkovski M, Vozari RR, Stühmer W, Bel ED (2013) Doxycycline restrains glia and confers neuroprotection in a 6-OHDA Parkinson model. Glia 61(7):1084–1100. doi:10.1002/glia.22496
Levkovitz Y, Fenchel D, Kaplan Z, Zohar J, Cohen H (2015) Early post-stressor intervention with minocycline, a second-generation tetracycline, attenuates post-traumatic stress response in an animal model of PTSD. Eur Neuropsychopharmacol 25(1):124–132. doi:10.1016/j.euroneuro.2014.11.012
Liu B, Wang S, Brenner M, Paton JF, Kasparov S (2008) Enhancement of cell-specific transgene expression from a Tet-Off regulatory system using a transcriptional amplification strategy in the rat brain. J Gene Med 10(5):583–592. doi:10.1002/jgm.1178
Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20(24):9104–9110
Markeljević J, Sarac H, Rados M (2011) Tremor, seizures and psychosis as presenting symptoms in a patient with chronic lyme neuroborreliosis (LNB). Coll Antropol 35(Suppl 1):313–318
McIver SR, Muccigrosso MM, Haydon PG (2012) The effect of doxycycline on alcohol consumption and sensitivity: consideration for inducible transgenic mouse models. Exp Biol Med 237(10):1129–1133. doi:10.1258/ebm.2012.012029 (Maywood)
McKernan DP, Dinan TG, Cryan JF (2009) “Killing the Blues”: a role for cellular suicide (apoptosis) in depression and the antidepressant response? Prog Neurobiol 88(4):246–263. doi:10.1016/j.pneurobio.2009.04.006
Mello BS, Monte AS, McIntyre RS, Soczynska JK, Custódio CS, Cordeiro RC, Chaves JH, Vasconcelos SM, Nobre HV Jr, de Sousa FC, Hyphantis TN, Carvalho AF, Macêdo DS (2013) Effects of doxycycline on depressive-like behavior in mice after lipopolysaccharide (LPS) administration. J Psychiatr Res 47(10):1521–1529. doi:10.1016/j.jpsychires.2013.06.008
Nagy EE, Rácz A, Urbán E, Terhes G, Berki T, Horváth E, Georgescu AM, Zaharia-Kézdi IE (2016) Diagnostic pitfalls in a young Romanian ranger with an acute psychotic episode. Neuropsychiatr Dis Treat 12:961–967. doi:10.2147/NDT.S103300
Paris JJ, Singh HD, Ganno ML, Jackson P, McLaughlin JP (2014) Anxiety-like behavior of mice produced by conditional central expression of the HIV-1 regulatory protein, Tat. Psychopharmacol 231(11):2349–2360. doi:10.1007/s00213-013-3385-1 (Berl)
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167
Porsolt RD, Anton G, Blavet N, Jalfre M (1978) Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 47(4):379–391
Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S (2005) Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol 192(2):348–356
Serchov T, Clement HW, Schwarz MK, Iasevoli F, Tosh DK, Idzko M, Jacobson KA, de Bartolomeis A, Normann C, Biber K, van Calker D (2015) Increased signaling via adenosine A1 receptors, sleep deprivation, imipramine, and ketamine inhibit depressive-like behavior via induction of homer1a. Neuron 87(3):549–562. doi:10.1016/j.neuron.2015.07.010
Shishkina GT, Kalinina TS, Berezova IV, Bulygina VV, Dygalo NN (2010) Resistance to the development of stress-induced behavioral despair in the forced swim test associated with elevated hippocampal Bcl-xl expression. Behav Brain Res 213(2):218–224. doi:10.1016/j.bbr.2010.05.003
Slattery DA, Cryan JF (2012) Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc 7(6):1009–1014. doi:10.1038/nprot.2012.044
Soczynska JK, Mansur RB, Brietzke E, Swardfager W, Kennedy SH, Woldeyohannes HO, Powell AM, Manierka MS, McIntyre RS (2012) Novel therapeutic targets in depression: minocycline as a candidate treatment. Behav Brain Res 235(2):302–317. doi:10.1016/j.bbr.2012.07.026
Stepanichev M, Dygalo NN, Grigoryan G, Shishkina GT, Gulyaeva N (2014) Rodent models of depression: neurotrophic and neuroinflammatory biomarkers. Biomed Res Int 2014:932757. doi:10.1155/2014/932757
Sultan S, Gebara E, Toni N (2013) Doxycycline increases neurogenesis and reduces microglia in the adult hippocampus. Front Neurosci 7:131. doi:10.3389/fnins.2013.00131
Tekin-Koruk S, Duygu F, Gursoy B, Karaagac L, Bayraktar M (2010) A rare case of seronegative neurobrucellosis. Ann Saudi Med 30(5):412–414. doi:10.4103/0256-4947.67084
Vaisburd S, Shemer Z, Yeheskel A, Giladi E, Gozes I (2015) Risperidone and NAP protect cognition and normalize gene expression in a schizophrenia mouse model. Sci Rep 5:16300. doi:10.1038/srep16300
Vicentini E, Arban R, Angelici O, Maraia G, Perico M, Mugnaini M, Ugolini A, Large C, Domenici E, Gerrard P, Bortner D, Mansuy IM, Mangiarini L, Merlo-Pich E (2009) Transient forebrain over-expression of CRF induces plasma corticosterone and mild behavioural changes in adult conditional CRF transgenic mice. Pharmacol Biochem Behav 93(1):17–24. doi:10.1016/j.pbb.2009.03.015
Vogt MA, Mallien AS, Pfeiffer N, Inta I, Gass P, Inta D (2016) Minocycline does not evoke anxiolytic and antidepressant-like effects in C57BL/6 mice. Behav Brain Res 301:96–101. doi:10.1016/j.bbr.2015.12.015
Walf AA, Frye CA (2007) The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2(2):322–328. doi:10.1038/nprot.2007.44
Yim CW, Flynn NM, Fitzgerald FT (1985) Penetration of oral doxycycline into the cerebrospinal fluid of patients with latent or neurosyphilis. Antimicrob Agents Chemother 28(2):347–348
Zhang GB, Feng YH, Wang PQ, Song JH, Wang P, Wang SA (2015) A study on the protective role of doxycycline upon dopaminergic neuron of LPS-PD rat model rat. Eur Rev Med Pharmacol Sci 19(18):3468–3474
Acknowledgements
This work was supported by the Russian Foundation for Basic Research N 15-04-07855 and Federal Agency of Scientific Organizations Program N 0324-2016-0002.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Shishkina, G.T., Lanshakov, D.A., Bannova, A.V. et al. Doxycycline Used for Control of Transgene Expression has its Own Effects on Behaviors and Bcl-xL in the Rat Hippocampus. Cell Mol Neurobiol 38, 281–288 (2018). https://doi.org/10.1007/s10571-017-0545-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-017-0545-6