Abstract
Mammalian target of rapamycin (mTOR) is a key regulator of metabolism, cell growth, and protein synthesis. Since decreased mTOR activity has been found to slow aging in many species, the aim of this study was to examine the activity of mTOR and its phosphorylated form in in vitro and in vivo models mimicking Alzheimer’s disease (AD), and investigate the potential pathway of PGC-1β in regulating mTOR expression. Primary neurons and N2a cells were treated with Aβ25–35, while untreated cells served as controls. The expression of mTOR, p-mTOR (Ser2448), and PGC-1β was determined with Western blotting and RT-PCR assay, and the translocation of mTOR was detected using confocal microscopy. Aβ25–35 treatment stimulated the translocation of mTOR from cytoplasm to nucleus, and resulted in elevated expression of mTOR and p-mTOR (Ser2448) and reduced PGC-1β expression. In addition, overexpression of PGC-1β was found to decrease mTOR expression. The results of this study demonstrate that Aβ increases the expression of mTOR and p-mTOR at the site of Ser2448, and the stimulation of Aβ is likely to depend on sirtuin 1, PPARγ, and PGC-1β pathway in regulating mTOR expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a common neurodegenerative disorder that impairs patients’ cognitive function and health (Querfurth and LaFerla 2010). This disorder is characterized by two hallmarks, β-amyloid (Aβ) accumulation and neurofibrillary tangles (Querfurth and LaFerla 2010), and it is widely accepted that cognitive impairments can be induced by accumulation of Aβ (Dries et al. 2012). In 2015, there were approximately 48 million people with AD worldwide (Alzheimer’s Association 2015), and it is predicted that over 106 million people are projected to have the disease by 2050 (Sloane et al. 2002).
Rapamycin, a mammalian target of rapamycin (mTOR) inhibitor, has shown activity against AD, suggesting the involvement of mTOR in AD pathogenesis (Cai et al. 2015; Richardson et al. 2015; Pei and Hugon 2008; Siman et al. 2015). mTOR, a conserved intracellular nutrient-sensing molecule, consists of two distinct signaling complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) (Pei and Hugon 2008). It was found that rapamycin inhibits only TORC1, which is composed of mTOR, mLST8, and raptor, while TORC2 is insensitive to rapamycin (Gaubitz et al. 2015; Loewith et al. 2002). As a serine/threonine protein kinase, mTOR exhibits its activity through phosphorylation of its substrates (Pei and Hugon 2008), and mTOR was found to be phosphorylated at Ser2448 via the PI3 kinase/Akt signaling pathway and autophosphorylated at Ser2481 (Chiang and Abraham 2005). Inhibition of mTOR activity has been found to slow aging (Harrison et al. 2009). In addition, Aβ was reported to increase the mTOR signaling, whereas reduced mTOR signaling decreased Aβ levels (Siman et al. 2015; Spilman et al. 2010), implicating a close interplay between mTOR signaling and Aβ (Caccamo et al. 2010).
Mitochondrial deficit is one of the main features of all major age-related neurodegenerative diseases (Chaturvedi and Flint Beal 2013). It has been demonstrated that the Aβ1–42 enters the mitochondria, leading to an increase in reactive oxygen species, interruption of the electron transport chain, and blockade of the ATP production (Chaturvedi and Flint Beal 2013). The peroxisome proliferator-activated receptor gamma coactivator 1 (PGC-1) family is crucial for the expression of nuclear genes encoding respiratory chain subunits (Wareski et al. 2009). Recent studies have indicated that overexpressing PGC-1 may improve mitochondrial respiratory function, and transgenic mice with impaired PGC-1 expression have neurodegenerative lesions (Hayashi et al. 2008; Ikeuchi et al. 2005; St-Pierre et al. 2006). PGC-1 family is composed of PGC-1α, PGC-1β, and PGC-1-related coactivators, and PGC-1α and PGC-1β have been recognized as the main regulators of mitochondrial biogenesis (Wareski et al. 2009). The ablation of PGC-1β was found to result in the constitutive activation of mTORC1 pathway in brain (Camacho et al. 2012; Yuan et al. 2016). In addition, rapamycin-induced mTOR inhibition is reported to greatly alleviate neurodegeneration caused by mitochondrial complex defects (Johnson et al. 2013). These findings prompted us to investigate the interactions between PGC-1β and mTOR in response to Aβ. PGC-1β has been widely recognized as a PPARγ coactivator, and sirtuin 1 (SIRT1) was also reported to activate PPARγ and PGC-1 (Kelly et al. 2009; Mudò et al. 2012; Qiang et al. 2012); however, whether these key regulators are involved in the mediation of mTOR expression is poorly known. The major purpose of this study, designed in in vitro and in vivo models mimicking AD, was to investigate the potential role of the PGC-1β, PPARγ, and SIRT1 pathway in regulating mTOR expression.
Materials and Methods
Ethical Consideration
All animal experimentation was performed in accordance with the recommendations of the Guidelines for the Care and Use of Laboratory Animals, Ministry of Science and Technology, People’s Republic of China [(2011)588]. This study was approved by the Ethics Review Committee of Fujian Medical University (Permission Number: FJMUERC2014-00089).
Animals
Eight-month old Tg2576 transgenic mice (APP transgenic mice carrying the Swedish mutation KM670/671NL) were purchased from Beijing Bio-ekon Biotechnology Co., Ltd. (Beijing, China).
Drugs
Aβ25–35 solution was prepared by dissolution of Aβ25–35 powder (Sigma-Aldrich; St. Louis, MO, USA) in ddH2O, and then incubated at 37 °C for 3 days. The drug solutions were stored at −80 °C for the subsequent experiments.
Cell Culture, Drug Treatment and Plasmid Transfection
Primary rat cortical neurons (PRCN) were prepared as described previously (Yuan et al. 2016) and maintained at 37 °C in a humidified incubator containing 9.5 % CO2. Mouse neuroblastoma Neuro-2a (N2a) cell line was purchased from the Cell Bank of Type Culture Collection, Chinese Academy of Sciences (Shanghai, China). N2a cells were cultured in 50 % Dulbecco’s modified Eagle’s medium (DMEM; Mediatech Inc.; Herndon, VA, USA)/50 % Opti-MEM (Gibco; Carlsbad, CA, USA) supplemented with 5 % fetal bovine serum. Primary neurons and N2a cells were treated with 25 μmol/L Aβ25–35 solution for an indicated time, while cells treated with the same volume of ddH2O served as controls. Transient transfections were performed using Lipofectamine 2000 (Invitrogen; Carlsbad, CA, USA) with the following plasmids: the expression vectors of pcDNA-PGC-1α, pcDNA-PGC-1β, pcDNA-PPARγ.WT, pcDNA-PPARγ.K268T/K293T, pcDNA-SIRT1.WT, and its mutant SIRT1.H355A.
Immunocytochemistry
Immunocytochemical staining on primary neurons and N2a cells was performed as previously reported (Ma et al. 2009). Briefly, primary neurons and N2a cells were rinsed with PBS and fixed with 4 % paraformaldehyde and 0.12 mol/L sucrose in PBS. N2a cells were permeabilized in 0.1 % Triton X-100 for 25 min. Fixed N2a cells were blocked with 5 % BSA in PBS for 1 h at room temperature and incubated in rabbit anti-mTOR (1:100; Cell Signaling Technology; Danvers, MA, USA) and goat anti-MAP-2 (1:100; Cell Signaling Technology; Danvers, MA, USA) at 4 °C overnight. Cells were then incubated in fluorescence-conjugated goat anti-rabbit IgG DyLight 594 (1:200; Life Technologies; Carlsbad, CA, USA) and DyLight 488 antibodies (1:200; Life Technologies; Carlsbad, CA, USA) at room temperature for 1 h, and counterstained with DAPI (Invitrogen; Carlsbad, CA, USA) to visualize nucleus.
Quantitative Real-Time RT-PCR
Total RNA was prepared by using TRIzol (Invitrogen; Carlsbad, CA, USA) according to the manufacturer’s instructions. Single-stranded cDNA was synthesized from 1 μg of total RNA using High-Capacity cDNA Reverse Transcription kits (Life Technologies; Carlsbad, CA, USA). Quantitative real-time PCR assay was performed with Power 2*SYBR Real-time PCR Premixture (BioTek Instruments, Inc.; Winooski, VT, USA) following the manufacturer’s protocols. Mouse Primers for mTOR (forward: 5′-GAAGAGCTGATTCGGGTAG-3′ and reverse: 5′- ACCATTCTTGTGCCTCCATT-3′), SIRT1 (forward: 5′- GACGGTATCTATGCTCGCCT-3′ and reverse: 5′-ATTCCTGCAACCTGCTCCAAG-3′), PGC-1β (forward: 5′- CTCCAGTTCCGGCTCCTC-3′ and reverse: 5′-CCCTCTGCTCTCACGTCTG-3′), and GAPDH (forward: 5′-AGCCTCCTTGATGGCCTCCTTG-3′ and reverse: 5′-AGAACATCATTCCCAGCAGC-3′) were used in the present studies. PCR amplification was performed in a 25 μl system containing 12.5 μl of 2 × Premix, 1 μl of each primer, 1 μl of cDNA template, and 9.5 μl of ddH2O under the following conditions: at 94 °C for 4 min; followed by 40 cycles at 94 °C for 15 s, and at 60.5 °C for 60 s; and finally at 60.5 °C for 30 s. Relative quantity of mRNA expression was calculated by using the 2−△△CT method.
Western Blotting Assay
Cells and cortex tissue lysates were prepared in RIPA lysis buffer (Ma et al. 2009). The same amount of total protein was resolved on 4–20 % Tris–Glycine gel, and the subsequent immunoblotting was performed as previously described (Ma et al. 2009). The primary antibodies used in this experiment included rabbit anti-mTOR (1:500; Cell Signaling Technology; Danvers, MA, USA), rabbit anti-Phospho-mTOR (Ser2448) (1:500; Cell Signaling Technology; Danvers, MA, USA), rabbit anti-PGC-1β (1:200; Santa Cruz Biotechnology, Inc.; Dallas, Texas, USA), rabbit anti-Myc-tag (1:1000; Cell Signaling Technology; Danvers, MA, USA), rabbit anti-PPARγ (1:1000; Abcam; Cambridge, MA, USA), rabbit anti-SIRT1 (1:1000; CST; Danvers, MA, USA), and rabbit anti-β-actin antibodies (1:5000; CST; Danvers, MA, USA).
Statistics
All measurement data were described as mean ± standard deviation (SD), and all statistical analyses were performed using the statistical software SPSS version 16.0 (SPSS, Inc.; Chicago, IL, USA). Student t test was employed to compare the difference between groups, and a P value <0.05 was considered statistically significant.
Results
Elevated mTOR and p-mTOR Expressions in Tg2576 Mice
Increasing evidences have shown that mTOR is involved in the pathogenesis of AD (Cai et al. 2015; Richardson et al. 2015; Pei and Hugon 2008; Siman et al. 2015). In addition, the levels of phosphorylated forms of mTOR are found to dramatically increase in AD and are strongly associated with tau and p tau (Alonso et al. 2001; Li et al. 2005). In this study, Western blotting assay showed a significant increase in the expression of mTOR and p-mTOR (at Ser2448) in the cortex tissues of Tg2576 mice, while a reduction was detected in the PGC-1β protein expression (Fig. 1a, b).
Western blotting analysis determines mTOR, p-mTOR, and PGC-1β protein expressions in cortex extracts of Tg2576 transgenic and control mice (n = 4). a Western blotting assay of mTOR, p-mTOR, and PGC-1β protein expressions, and β-actin serves as a loading control. b Comparison of mTOR, p-mTOR, and PGC-1β protein expressions in the cortex extracts between Tg2576 transgenic and control mice. Data are presented as mean ± SD, n = 4, *P < 0.05
Aβ25–35 Increases mTOR Expression and mTOR Phosphorylation in PRCN and N2a Cells
Overwhelming evidence supports Aβ oligomers as an early trigger of synaptic damage and cognitive impairment in AD (Ferreira et al. 2015; Viola and Klein 2015; Zahs and Ashe 2013). In this study, we determined mTOR and p-mTOR (at Ser2448) expressions in Aβ25–35-treated PRCN and N2a cells. Western blotting analysis revealed a significant increase in mTOR and p-mTOR expressions 6 h post-treatment, and a reduction in PRCN 24 h post-treatment (Fig. 2a). Compared to untreated PRCN, mTOR expression increased 2.3-fold and p-mTOR expression increased 2.5-fold 6 h post-treatment (Fig. 2b).
Western blotting analysis determines mTOR and p-mTOR protein expressions in PRCN and N2a cells. a Western blotting assay of mTOR and p-mTOR protein expressions in PRCN 0, 1, 6, and 24 h post-treatment with 25 μmol/L Aβ25–35. b Western blotting assay of mTOR and p-mTOR protein expressions in PRCN 6 h post-treatment with 25 μmol/L Aβ25–35, while untreated tissues serve as controls. c Western blotting assay of mTOR, p-mTOR, SIRT1, and PGC-1β protein expressions in N2a cells 6 h post-treatment with 25 μmol/L Aβ25–35, while untreated cells serve as controls. d mRNA expression of mTOR, SIRT1, and PGC-1β in N2a cells. β-actin serves as an internal reference. Data are expressed as mean ± SD, n = 3. *P < 0.05, **P < 0.01
Both mouse cortical tissue and primary cultured rat cortical neurons contain neurons, astrocytes, microglia, and some other cells. Astrocytes can protect neurons from Aβ via PGC-1/SIRT1 pathways (Aguirre-Rueda et al. 2015). To probe the mTOR activity in neurons following exposure to Aβ stimulus, we examined mTOR expression in N2a cells at both translational and transcriptional levels. Western blotting analysis and RT-PCR assay showed that Aβ25–35 treatment up-regulated mTOR mRNA expression, resulting in a 2.3-fold increase in mTOR and a 2.8-fold increase in p-mTOR protein expression 6 h post-treatment in relative to untreated controls, and Aβ25–35 treatment caused a reduction in PGC-1β expression at both translational and transcriptional levels. However, Aβ25–35 treatment had no clear-cut effect on SIRT1 expression (Fig. 2c, d).
Aβ25–35 Induces Nucleocytoplasmic Translocation of mTOR and Blocks Neurite Growth In Vitro
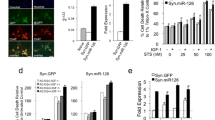
mTOR is located in lysosome, plasma membrane, peroxisome, mitochondria, and nucleus (Pei and Hugon 2008). In this study, confocal microscopy revealed that mTOR protein was distributed throughout the cytoplasm of untreated PRCN, while mTOR protein translocation into nucleus was seen in 50 % percent of the Aβ25–35-treated PRCN 1 h post-treatment (Fig. 3a). In addition, mTOR was found to be predominantly accumulated in the cytoplasm of untreated N2a cells, and partially localized in the nucleus of N2a cells following exposure to 25 μmol/L Aβ25–35 for 6 h (Fig. 3b). These findings suggest that Aβ mediates mTOR translocation in neurons.
Effect of Aβ25–35 on nuclear translocation of mTOR in PRCN and N2a cells. a PRCN were labeled with anti-mTOR antibody and anti-MAP2 antibody 1 h post-treatment with 25 μmol/L Aβ25–35, and then visualized under a confocal laser scanning microscope. Scale bar 20 μm. b Following exposure to 25 μmol/L Aβ25–35 for 6 h, N2a cells were labeled with anti-mTOR antibody, and the nuclei were visualized by DAPI counterstain. Scale bar 10 μm
Effect of PGC-1β on mTOR Requires Deacetylation by SIRT1
mTORC1 activity has been detected in mitochondria-defective neurons (Johnson et al. 2013; Zheng et al. 2016), and PGC-1 coactivators were reported to regulate mitochondrial biogenesis (Villena 2015). In the present study, we found elevated mTOR expression and reduced PGC-1β expression in response to Aβ stimulus (Figs. 1, 2). We then investigated the role of PGC-1β in regulating mTOR. In N2a cells, overexpression of PGC-1α caused down-regulation of mTOR protein expression, while overexpression of PGC-1β caused an approximately threefold reduction in mTOR protein expression (Fig. 4a).
PGC-1β suppression of mTOR requires SIRT1 deacetylase activity. a Effect of PGC-1 on mTOR suppression. N2a cells are transfected with Myc-tagged PGC-1α, Myc-tagged PGC-1β alone, or in combination with SIRT.H355A plasmid, while cells transfected with vector alone serve as controls. The mTOR, Myc, and SIRT1 protein expressions were determined in N2a cells 48 h post-transfection. b Effects of wild-type and nonacetylated PPARγ (K268T and K293T) overexpression on mTOR expression in N2a cells. Protein levels were detected in N2a cells 48 h after transient transfection. c Effects of wild-type and a dominant-negative mutant SIRT1 (SIRT.H355A) overexpression on mTOR expression in N2a cells. Protein levels were detected in N2a cells 48 h after transient transfection; d Effects of Myc-tagged PGC-1β alone or in combination with wild-type SIRT plasmid on mTOR expression in N2a cells. Protein levels were detected in N2a cells 48 h after transient transfection, while β-actin serves as a loading control. mTOR protein level relative to β-actin in the vehicle-treated group was set to 1; Values are presented as mean ± SD, n = 3, *P < 0.05, **P < 0.01
Since PGC-1 has been identified as a coactivator for PPARγ, SIRT1 was reported to activate PPARγ by deacetylation on K268 and K293 in adipocytes (Qiang et al. 2012). Therefore, we investigated the role of PPARγ and its deacetylation in mTOR expression. Western blotting analysis showed that overexpression of nonacetylated PPARγ (K268T and K293T), an active form of PPARγ, led to a 40 % reduction in mTOR transcription (Fig. 4b). Surprisingly, overexpression of PPARγ (wild-type) had no remarkable effect on mTOR expression.
Recently, SIRT1 was also reported to activate PGC-1β via deacetylation (Kelly et al. 2009). In the present study, we found overexpression of SIRT1 suppressed mTOR expression, while deacetylase-inactive H355A SIRT1 had an opposite effect on N2a cells (Fig. 4c). Notably, the coexpression of PGC-1β and SIRT1.H355A plasmid constructs in N2a cells alleviated the suppression on mTOR protein expression mediated by PGC-1β (Fig. 4a), and coexpression of PGC-1β and wild-type SIRT1 exerted a further additive effect (Fig. 4d).
Discussion
AD, the most common form of dementia, is a complex disease characterized by senile plaques, neurofibrillary tangles, and neuronal loss (Querfurth and LaFerla 2010). Senile plaques are mainly composed of Aβ fibrils. Fibrillar Aβ is preceded by several conformational states, including dimers, oligomers, and an insoluble fibrillar conformation. However, increasing evidence suggests that oligomeric Aβ may be more important than insoluble fibrillar Aβ in AD etiopathology (Deshpande et al. 2006). In this study, we used oligomeric Aβ, the soluble forms to treat cells and animals.
Aβ has been proved to cause inflammation, oxidative stress, and mitochondrial dysfunction (Trushina and McMurray 2007; Vallés et al. 2008). Increasing evidence has demonstrated that rapamycin, an mTOR inhibitor, may help to relieve neurodegeneration in animal models of AD (Siman et al. 2015; Zheng et al. 2016). Our data clearly showed that Aβ25–35 activated mTOR and p-mTOR (at Ser2448) expressions both in vitro and in vivo. Our data revealed an over two-fold higher increase in mTOR and p-mTOR expressions 6 h post-treatment, and a reduction in PRCN 24 h post-treatment. It seems that the role of mTOR activation is very complex. In this study, we found that Aβ25–35-treated neurons died in a dose- and time-dependent manner (data not shown). It is considered that mTOR may be activated and deactivated during the progress of neuronal apoptosis, dependent on the duration of exposure to Aβ25–35. It is therefore hypothesized that rapamycin may protect neuron from death by inhibiting mTOR phosphorylation at the early stage. Our data indicate that Aβ25–35 up-regulated mTOR and p-mTOR expressions in PRCN and N2a cells. However, it seems that the increased mTOR phosphorylation at the S2448 site is associated with total mTOR activation based on the ratio of p-mTOR to total mTOR.
mTOR protein is expressed predominantly in cytoplasm, and the major function of mTOR pathway is mTOR kinase activity toward its cytoplasmic substrates. However, nuclear mTOR has been recently found in rhabdomyosarcomas, human fibroblasts, and HCT8 colon carcinoma cells, and leptomycin B was reported to modulate mTOR nuclear import in HEK293 cells (Kim and Chen 2000; Zhang et al. 2002). In the current study, confocal microscopy showed that mTOR was localized exclusively in neuronal cytoplasm at baseline. However, accumulation of mTOR was observed in nucleus and perinuclear area of PRCN and N2a cells treated with Aβ25–35, suggesting the translocation of mTOR into the nucleus of neurons following exposure to Aβ25–35. This is the first report of mTOR nuclear localization in response to Aβ. Our findings demonstrate the potential role of nuclear mTOR in neurodegeneration; however, further studies to investigate the mechanisms underlying the nuclear translocation of p-mTOR (at Ser2448) seem justified.
Mitochondrial dysfunction is frequently observed in neurodegenerative diseases (Chaturvedi and Flint Beal 2013). PGC-1 coactivators are reported to regulate mitochondrial biogenesis in neurons (Wareski et al. 2009). PGC-1α expression was previously reported to decrease in AD brain (Qin et al. 2009); however, the PGC-1β expression remains unknown in AD to date. In this study, we observed decreased PGC-1β expression in Tg2576 mice and N2a cells treated with Aβ. However, our findings were obtained from cell and animal experiments; further human studies are required to validate the conclusion. It was reported that deletion of PGC-1β led to activate mTOR in mouse cortical neurons (Ikeuchi et al. 2005). We speculate that increased PGC-1β expression may down-regulate mTOR activity. To test this hypothesis, we investigated the effect of PGC-1α and PGC-1β overexpressions on mTOR expression in this study. Western blotting analysis revealed that PGC-1a and PGC-1β down-regulated mTOR expression, and PGC-1β appeared to be a more important regulator for mTOR.
In the present study, we observed that overexpression of mutant PPARγ at K268T and K293T significantly suppressed mTOR expression, as revealed by Western blotting assay. Deacetylation from two residues, SIRT1 is likely to be responsible for the PPARγ suppression on mTOR expression. SIRT1 has a role in deacetylation of target proteins, and significant decreases in SIRT1 protein expression are detected in AD patients (Kumar et al. 2013). We did not detect a significant decrease of SIRT1 in N2a cells following Aβ25–35 treatment; on the contrary, a slightly increased SIRT1 expression was detected in our findings. SIRT1 knockout was found to cause an increase in mTOR activation in mouse embryonic fibroblast (Ghosh et al. 2010), suggesting that decreased SIRT1 may trigger an increase in mTOR activity. Our findings showed overexpressing wild-type SIRT1 suppressed mTOR expression, while mutant SIRT1 (an inactive form) up-regulated mTOR activity, which was consistent with previous report (Ghosh et al. 2010). Interestingly, the effect of PGC-1β on mTOR expression was found to require the deacetylation by SIRT1, consistent to the previous report that SIRT1 is the upstream regulator of PPARγ and PGC-1 (Wang et al. 2013).
In conclusion, the results of this study demonstrate, for the first time, the interplay among PGC-1β, PPARγ, SIRT1, and mTOR. Although the exact regulatory network and the underlying mechanisms require further investigations, our work may provide new insights into the identification of molecular targets for AD.
References
Aguirre-Rueda D, Guerra-Ojeda S, Aldasoro M, Iradi A, Obrador E, Ortega A, Mauricio MD, Vila JM, Valles SL (2015) Astrocytes protect neurons from Aβ1-42 peptide-induced neurotoxicity increasing TFAM and PGC-1 and decreasing PPAR-γ and SIRT-1. Int J Med Sci 12(1):48–56. doi:10.7150/ijms.10035
Alonso A, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K (2001) Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA 98(12):6923–6928. doi:10.1073/pnas.121119298
Alzheimer’s Association (2015) 2015 Alzheimer’s disease facts and figures. Alzheimers dement 11:332–384. doi:10.1016/j.jalz.2015.02.003
Caccamo A, Majumder S, Richardson A, Strong R, Oddo S (2010) Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and tau: effects on cognitive impairments. J Biol Chem 285(17):13107–13120. doi:10.1074/jbc.M110.100420
Cai Z, Chen G, He W, Xiao M, Yan LJ (2015) Activation of mTOR: a culprit of Alzheimer’s disease? Neuropsychiatr Dis Treat 11:1015–1030. doi:10.2147/NDT.S75717
Camacho A, Rodriguez-Cuenca S, Blount M, Prieur X, Barbarroja N, Fuller M, Hardingham GE, Vidal-Puig A (2012) Ablation of PGC1 beta prevents mTOR dependent endoplasmic reticulum stress response. Exp Neurol 237(2):396–406. doi:10.1016/j.expneurol.2012.06.031
Chaturvedi RK, Flint Beal M (2013) Mitochondrial diseases of the brain. Free Radic Biol Med 63:1–29. doi:10.1016/j.freeradbiomed.2013.03.018
Chiang GG, Abraham RT (2005) Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem 280(27):25485–25490. doi:10.1074/jbc.M501707200
Deshpande A, Mina E, Glabe C, Busciglio J (2006) Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci 26(22):6011–6018. doi:10.1523/JNEUROSCI.1189-06.2006
Dries DR, Yu G, Herz J (2012) Extracting β-amyloid from Alzheimer’s disease. Proc Natl Acad Sci USA 109(9):3199–3200. doi:10.1073/pnas.1121560109
Ferreira ST, Lourenco MV, Oliveira MM, De Felice FG (2015) Soluble amyloid-β oligomers as synaptotoxins leading to cognitive impairment in Alzheimer’s disease. Front Cell Neurosci 9:191. doi:10.3389/fncel.2015.00191
Gaubitz C, Oliveira TM, Prouteau M, Leitner A, Karuppasamy M, Konstantinidou G, Rispal D, Eltschinger S, Robinson GC, Thore S, Aebersold R, Schaffitzel C, Loewith R (2015) Molecular basis of the rapamycin insensitivity of target of rapamycin complex 2. Mol Cell 58(6):977–988. doi:10.1016/j.molcel.2015.04.031
Ghosh HS, McBurney M, Robbins PD (2010) SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One 5(2):e9199. doi:10.1371/journal.pone.0009199
Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460(7253):392–395. doi:10.1038/nature08221
Hayashi Y, Yoshida M, Yamato M, Ide T, Wu Z, Ochi-Shindou M, Kanki T, Kang D, Sunagawa K, Tsutsui H, Nakanishi H (2008) Reverse of age-dependent memory impairment and mitochondrial DNA damage in microglia by an overexpression of human mitochondrial transcription factor in mice. J Neurosci 28(34):8624–8634. doi:10.1523/JNEUROSCI.1957-08.2008
Ikeuchi M, Matsusaka H, Kang D, Matsushima S, Ide T, Kubota T, Fujiwara T, Hamasaki N, Takeshita A, Sunagawa K, Tsutsui H (2005) Overexpression of mitochondrial transcription factor A ameliorates mitochondrial deficencies and cardiac failure after myocardial infarction. Circulation 112(5):683–690. doi:10.1161/CIRCULATIONAHA.104.524835
Johnson SC, Yanos ME, Kayser EB, Quintana A, Sangesland M, Castanza A, Uhde L, Hui J, Wall VZ, GagnidzeA OhK, Wasko BM, Ramos FJ, Palmiter RD, Rabinovitch PS, Morgan PG, Sedensky MM, Kaeberlein M (2013) mTOR inhibition alleviates mitochondrial disease in a mouse model of leigh syndrome. Science 342:1524–1528. doi:10.1126/science.1244360
Kelly TJ, Lerin C, Haas W, Gygi SP, Puigserver P (2009) GCN5-mediated transcriptional control of the metabolic coactivator PGC-1beta through lysine acetylation. J Biol Chem 284(30):19945–19952. doi:10.1074/jbc.M109.015164
Kim JE, Chen J (2000) Cytoplasmic-nuclear shuttling of FKBP12- rapamycin associated protein is involved in rapamycin-sensitive signaling and translation initiation. Proc Natl Acad Sci USA 97(27):14340–14345. doi:10.1073/pnas.011511898
Kumar R, Chaterjee P, Sharma PK, Singh AK, Gupta A, Gill K, Tripathi M, Dey AB, Dey S (2013) Sirtuin1: a promising serum protein marker for early detection of Alzheimer’s disease. PLoS One 8(4):e61560. doi:10.1371/journal.pone.0061560
Li X, Alafuzoff I, Soininen H, Winblad B, Pei JJ (2005) Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer’s disease brain. Febs J 272(16):4211–4220. doi:10.1111/j.1742-4658.2005.04833.x
Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN (2002) Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 10(3):457–468. doi:10.1016/S1097-2765(02)00636-6
Ma T, Zhao Y, Kwak YD, Yang Z, Thompson R, Luo Z, Xu H, Liao FF (2009) Statin’s excitoprotection is mediated by sAPP and the subsequent attenuation of calpain-induced truncation events, likely via rho-ROCK signaling. J Neurosci 29(36):11226–11236. doi:10.1523/JNEUROSCI.6150-08.2009
Mudò G, Mäkelä J, Di Liberto V, Tselykh TV, Olivieri M, Piepponen P, Eriksson O, Mälkiä A, Bonomo A, Kairisalo M, Aguirre JA, Korhonen L, Belluardo N, Lindholm D (2012) Transgenic expression and activation of PGC-1α protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell Mol Life Sci 69(7):1153–1165. doi:10.1007/s00018-011-0850-z
Pei JJ, Hugon J (2008) mTOR-dependent signalling in Alzheimer’s disease. J Cell Mol Med 12(6B):2525–2532. doi:10.1111/j.1582-4934.2008.00509.x
Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, Accili D (2012) Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of pparγ. Cell 150(3):620–632. doi:10.1016/j.cell.2012.06.027
Qin W, Haroutunian V, Katsel P, Cardozo CP, Ho L, Buxbaum JD, Pasinetti GM (2009) PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia. Arch Neurol 66(3):352–361. doi:10.1001/archneurol.2008.588
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362(4):329–344. doi:10.1056/NEJMra0909142
Richardson A, Galvan V, Lin AL, Oddo S (2015) How longevity research can lead to therapies for Alzheimer’s disease: the rapamycin story. Exp Gerontol 68:51–58. doi:10.1016/j.exger.2014.12.002
Siman R, Cocca R, Dong Y (2015) The mTOR inhibitor rapamycin mitigates perforant pathway neurodegeneration and synapse loss in a mouse model of early-stage Alzheimer-type tauopathy. PLoS One 10(11):e0142340. doi:10.1371/journal.pone.0142340
Sloane PD, Zimmerman S, Suchindran C, Reed P, Wang L, Boustani M, Sudha S (2002) The public health impact of Alzheimer’s disease, 2000–2050: potential implication of treatment advances. Annu Rev Public Health 23:213–231. doi:10.1146/annurev.publhealth.23.100901.140525
Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V (2010) Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One 5(4):e9979. doi:10.1371/journal.pone.0009979
St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM (2006) Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127(2):397–408. doi:10.1016/j.cell.2006.09.024
Trushina E, McMurray CT (2007) Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience 145(4):1233–1248. doi:10.1016/j.neuroscience.2006.10.056
Vallés SL, Borrás C, Gambini J, Furriol J, Ortega A, Sastre J, Pallardó FV, Viña J (2008) Oestradiol or genistein rescues neurons from amyloid beta-induced cell death by inhibiting activation of p38. Aging Cell 7(1):112–118. doi:10.1111/j.1474-9726.2007.00356.x
Villena JA (2015) New insights into PGC-1 coactivators: redefining their role in the regulation of mitochondrial function and beyond. Febs J 282(4):647–672. doi:10.1111/febs.13175
Viola KL, Klein WL (2015) Amyloid β oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathol 129(2):183–206. doi:10.1007/s00401-015-1386-3
Wang R, Li JJ, Diao S, Kwak YD, Liu L, Zhi L, Büeler H, Bhat NR, Williams RW, Park EA, Liao FF (2013) Metabolic stress modulates Alzheimer’s β-secretase gene transcription via SIRT1-PPARγ-PGC-1 in neurons. Cell Metab 17(5):685–694. doi:10.1016/j.cmet.2013.03.016
Wareski P, Vaarmann A, Choubey V, Safiulina D, Liiv J, Kuum M, Kaasik A (2009) PGC-1{alpha} and PGC-1{beta} regulate mitochondrial density in neurons. J Biol Chem 284(32):21379–21385. doi:10.1074/jbc.M109.018911
Yuan Y, Wang Y, Hu FF, Jiang CY, Zhang YJ, Yang JL, Zhao SW, Gu JH, Liu XZ, Bian JC, Liu ZP (2016) Cadmium activates reactive oxygen species-dependent AKT/mTOR and mitochondrial apoptotic pathways in neuronal cells. Biomed Environ Sci 29(2):117–126. doi:10.3967/bes2016.013
Zahs KR, Ashe KH (2013) β-Amyloid oligomers in aging and Alzheimer’s disease. Front Aging Neurosci 5:28. doi:10.3389/fnagi.2013.00028
Zhang X, Shu L, Hosoi H, Murti GK, Houghton PJ (2002) Predominant nuclear localization of mammalian target of rapamycin in normal and malignant cells in culture. J Biol Chem 277(31):28127–28134. doi:10.1074/jbc.M202625200
Zheng X, Boyer L, Jin M, Kim Y, Fan W, Bardy C, Berggren T, Evans RM, Gage FH, Hunter T (2016) Alleviation of neuronal energy deficiency by mTOR inhibition as a treatment for mitochondria-related neurodegeneration. Elife 5:e13378. doi:10.7554/eLife13378
Acknowledgments
We would like to express our sincere thanks to Dr. Francesca-Fang Liao from University of Tennessee Health Science Center for kindly providing us pcDNA-PPARγ.WT, pcDNA-PPARγ.K268T/K293T, pcDNA-SIRT1.WT, and its mutant SIRT1.H355A. This study was supported by the Grants from the Academic Development Funds for the Professors of Fujian Medical University (Grant No. JS/14002) and the Doctoral Starting-up Foundation of Fujian Medical University (Grant No. 2009bs002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Liu, YC., Gao, XX., Zhang, ZG. et al. PPAR Gamma Coactivator 1 Beta (PGC-1β) Reduces Mammalian Target of Rapamycin (mTOR) Expression via a SIRT1-Dependent Mechanism in Neurons. Cell Mol Neurobiol 37, 879–887 (2017). https://doi.org/10.1007/s10571-016-0425-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-016-0425-5